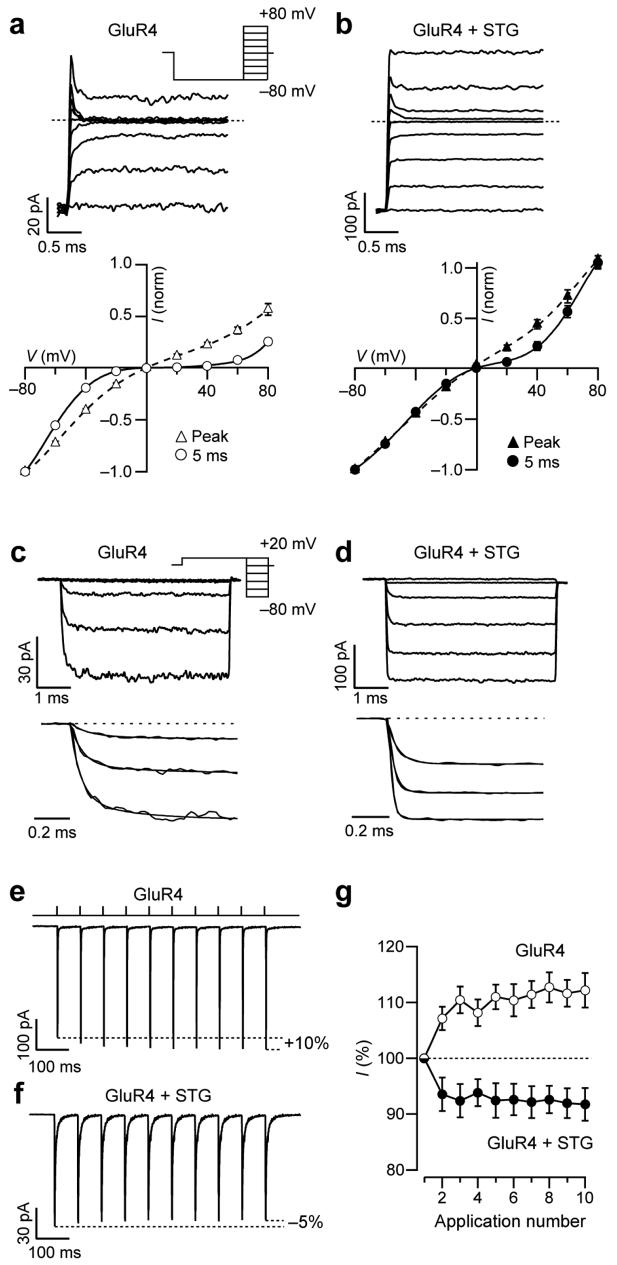
Figure 4.
Stargazin alters the time course of spermine block and eliminates frequency-dependent facilitation of Ca2+-permeable GluR4 AMPARs. (a, b) Top, representative responses of homomeric GluR4 AMPARs, alone (a) or with stargazin (b), to depolarizing voltage steps in the presence of 1 mM glutamate. After a hyperpolarizing pre-pulse to −80 mV, outside-out patches were stepped to +80 mV in 20 mV increments (inset). Cyclothiazide (50 μM) was included in the extracellular medium to minimize desensitization, and the ‘intracellular’ solution contained spermine (30 μM). Broken line indicates zero current. Relaxations following voltage steps were greatly diminished with stargazin. Bottom, corresponding I-V relationships for peak and steady-state (5 ms) currents (n = 7 cells without and n = 5 cells with stargazin). (c, d) Top, relaxation responses to hyperpolarizing voltage steps in the presence of 1mM glutamate applied to patches containing GluR4 (c) or GluR4 plus stargazin (d). The potential was stepped from +20 mV to −80 mV in −20 mV increments (after a pre-pulse from 0 to +20 mV; inset). Cyclothiazide and spermine were included as in a,b. Bottom, onset of the currents; those recorded at −80 to −40 mV were fitted with exponentials. The kinetics of the currents following voltage steps was much faster in the presence of stargazin. (e, f) Currents activated by a train of glutamate pulses (1 mM, 1 ms) applied at 14 Hz to patches expressing GluR4 (e) or GluR4 plus stargazin (f). Currents from GluR4 alone increased in amplitude during the train, whereas those from GluR4 plus stargazin showed depression (−60 mV; 10 μM intracellular spermine). Traces are averages of 60 or 100 trials. (g) Pooled data from cells expressing GluR4 alone (n = 16) or GluR4 with stargazin (n = 9; P <0.0001 by two-way repeated measures ANOVA).