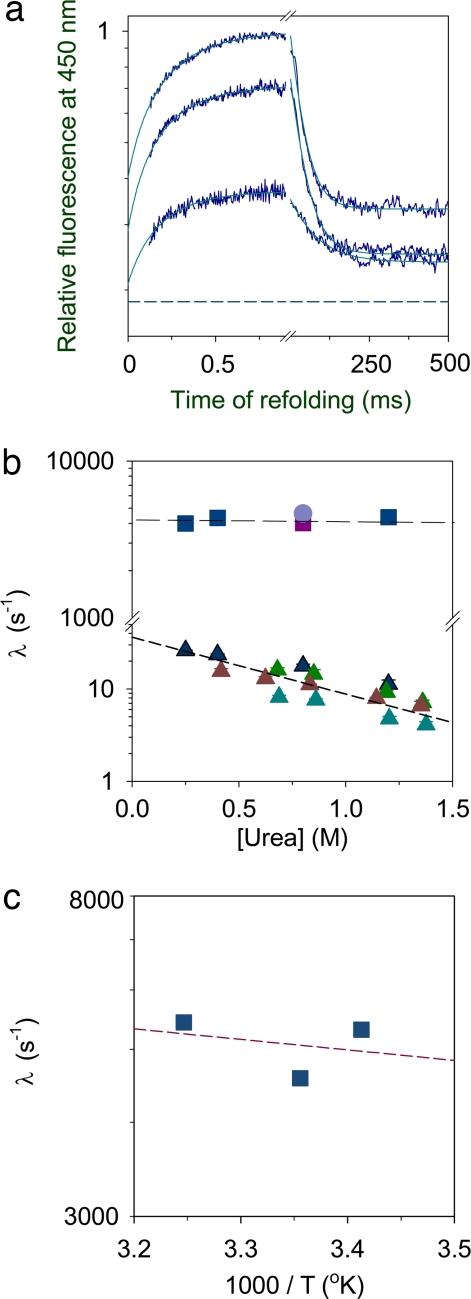
Fig. 3.
Dependence of the folding kinetics on urea concentration. (a) Representative very fast kinetic traces in final urea concentrations of 0.25 M (top trace), 0.4 M (middle trace), and 1.2 M (bottom trace) are shown. The very fast phase corresponding to the sub-ms exponential rise of the ANS fluorescence was captured by using the microsecond mixer. The >1-ms part of the data was obtained by using a stopped-flow mixer under identical conditions. The dashed line represents the unfolded protein baseline. The y axis is shown on a log scale for a comparison of the kinetics in different urea concentrations. The data shown here are normalized to a value of 1 for the t = ∞ signal of the sub-ms kinetic refolding trace obtained in 0.25 M urea. The lines through the data are fits to a two-exponential equation. (b) Comparison of the kinetics monitored by different probes. Shown are the very fast rate constants of the sub-ms folding reaction (blue squares) and the fast rate constants (blue triangles) of the stopped-flow-monitored millisecond folding reaction, both obtained from the measurement of the ANS-fluorescence-monitored refolding kinetics; the very fast rate constant of the ANS-fluorescence-monitored sub-ms refolding reaction in 0.8 M urea in the presence of a 4-fold-higher concentration of ANS (4 mM) (blue circle) and a 2-fold-higher concentration of the protein (40 μM) (magenta square); and the observed fast rate constants monitored by Trp fluorescence (brown triangles), far-UV CD (green triangles), and FRET (teal triangles). In all cases, the errors bars indicating the spreads in the values, which were determined from two or more repetitions of the experiments, are smaller than the sizes of the symbols. (c) Arrhenius plot showing the temperature dependence of the apparent rate constant of the very fast phase. The slope of the plot yields an apparent activation energy of 0.6 kcal·mol−1.