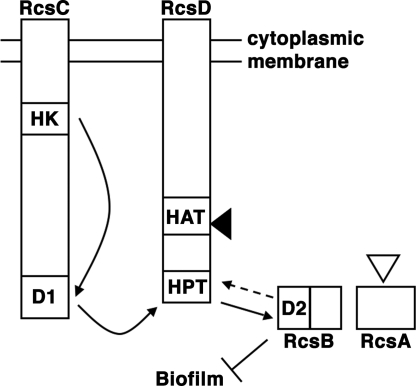
Fig. 1.
Model of Rcs phosphorelay signaling. The histidine kinase domain (HK) of RcsC autophosphorylates, after which phosphate is transferred intramolecularly to aspartate in a receiver domain (D1). Next, phosphate is transferred intermolecularly from D1 to the histidine phosphotransfer domain (HPT) of RcsD. Last, phosphate is transferred from HPT to the RcsB receiver domain (D2). Biofilms are repressed by RcsB, and repression is increased by functional RcsA, as described in Results. Solid arrows show phosphate transfers that are well established experimentally (13, 14); dashed arrow indicates hypothesized phosphatase activity by RcsC, RcsD, or both (13, 16, 17). The histidine kinase-like ATPase (HAT) domain of RcsD does not participate in phosphotransfer. Filled arrowhead, site of frameshift in Y. pestis RcsD. Open arrowhead, site of inactivating insertion in Y. pestis RcsA.