Abstract
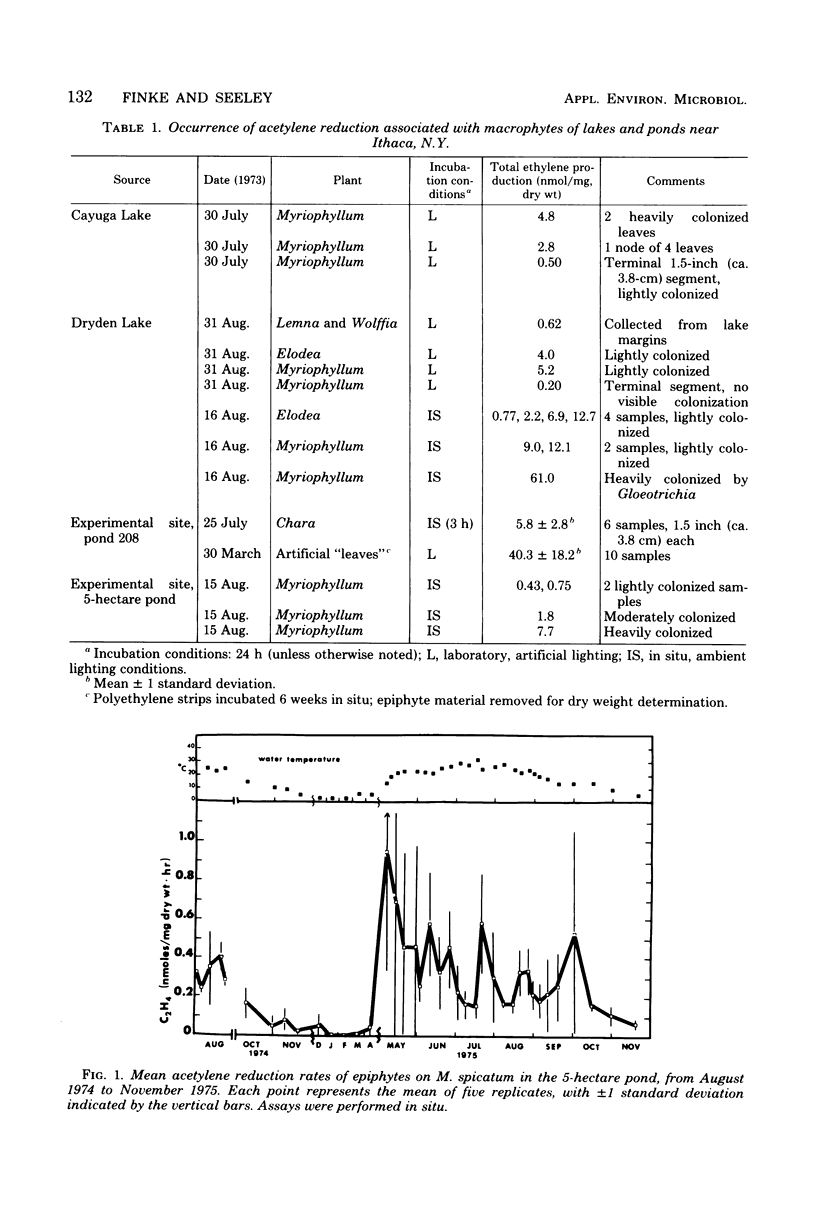
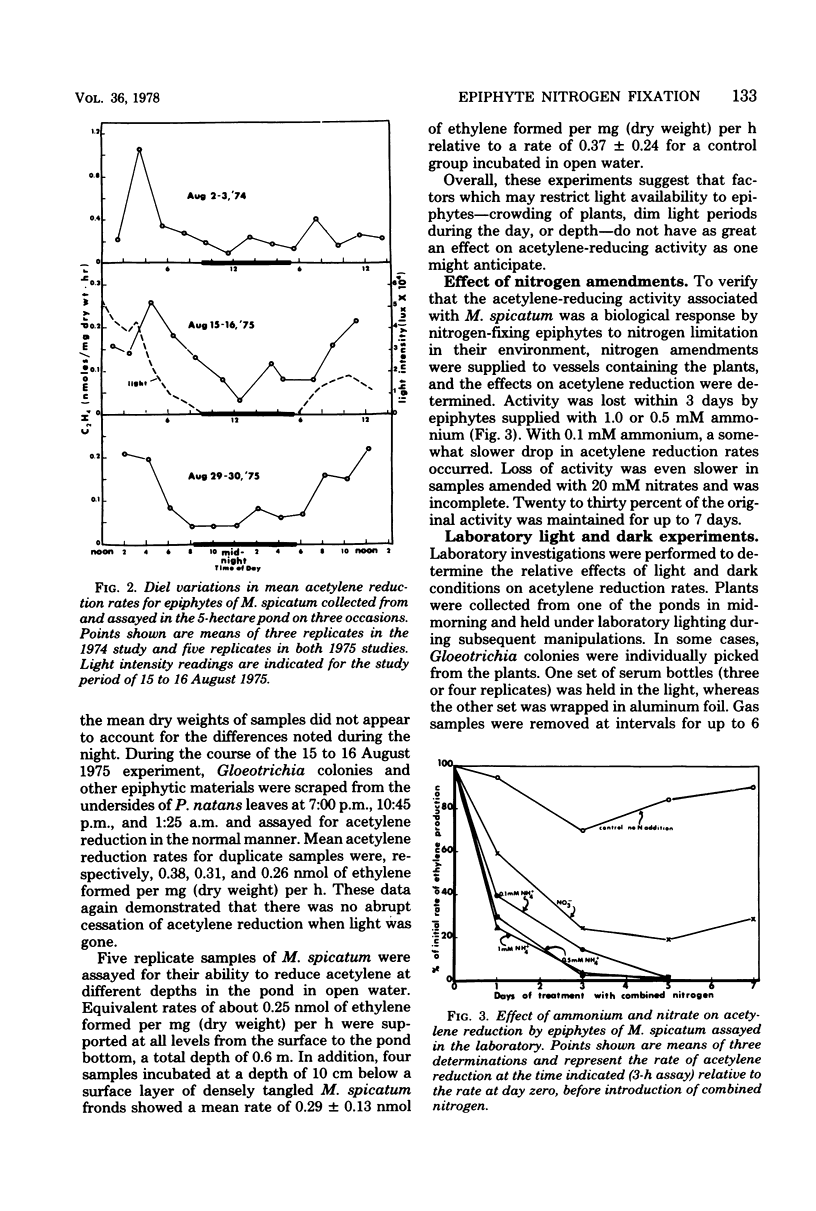
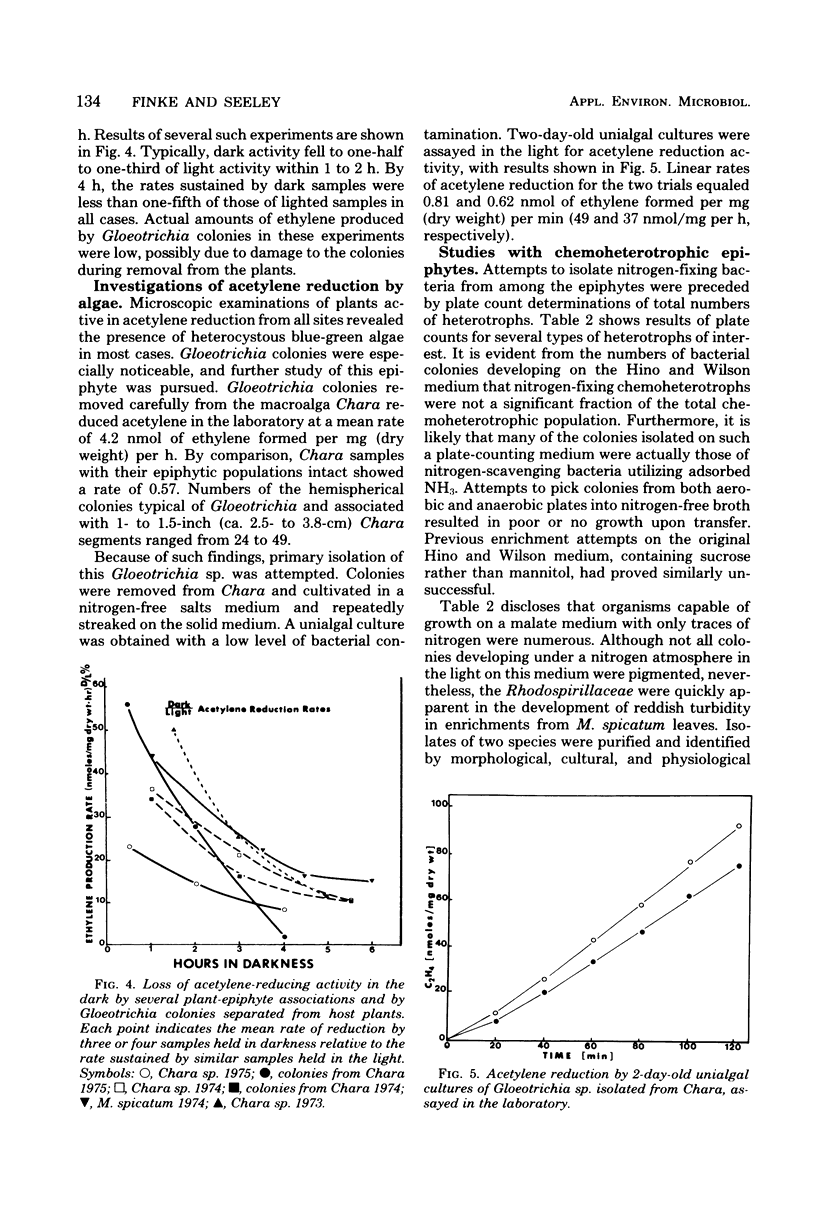
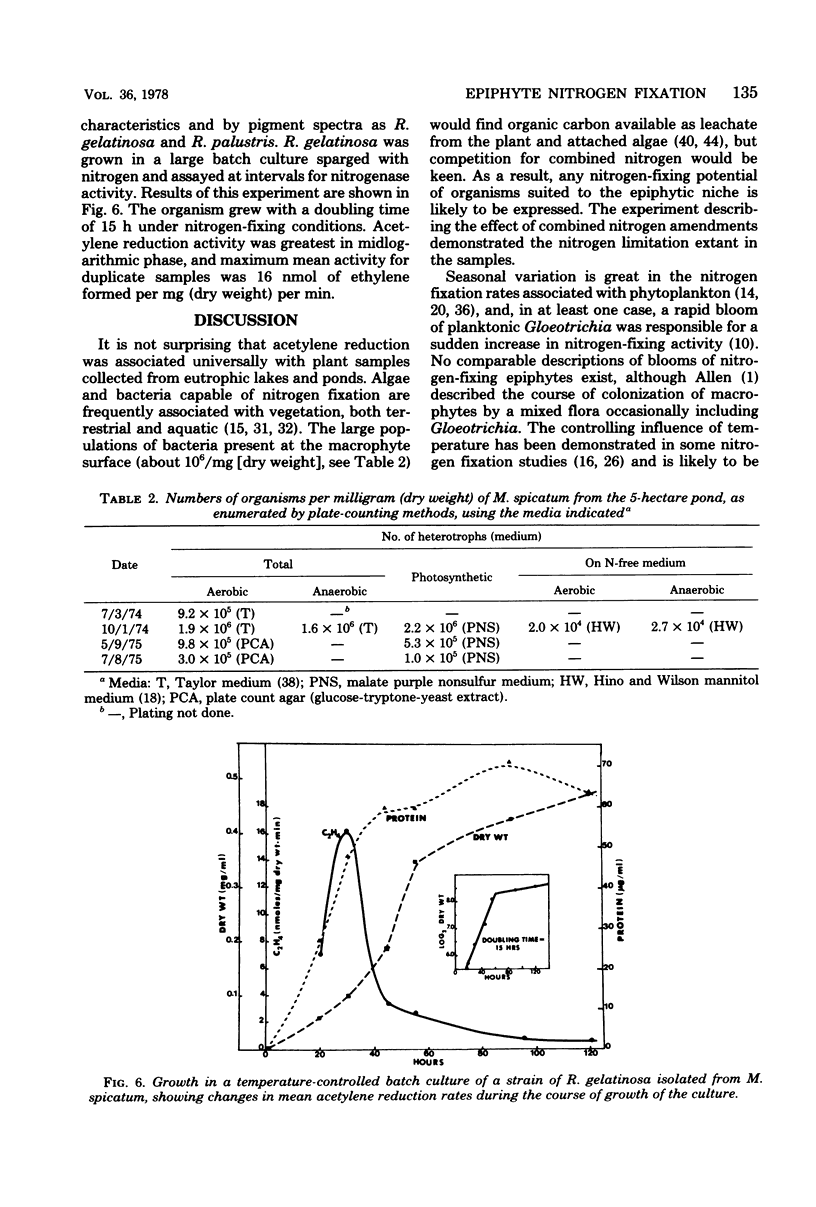
The involvement of epiphytic microorganisms in nitrogen fixation was investigated in a shallow freshwater pond near Ithaca, N.Y. The acetylene reduction technique was used to follow diel and seasonal cycles of nitrogen fixation by epiphytes of Myriophyllum spicatum. Acetylene-reducing activity was maximal between noon and 6 p.m., but substantial levels of activity relative to daytime rates continued through the night. Experiments with the seasonal course of activity showed a gradual decline during the autumn months and no activity in January or February. Activity commenced in May, with an abrupt increase to levels between 0.45 and 0.95 nmol of ethylene formed per mg (dry weight) of plant per h. Through most of the summer months, mean rates of acetylene reduction remained between 0.15 and 0.60 nmol/mg (dry weight) per h. It was calculated from diel and seasonal cycles that, in the pond areas studied, epiphytes were capable of adding from 7.5 to 12.5 μg of N per mg of plant per year to the pond. This amount is significant relative to the total amount of nitrogen incorporated into the plant. Blue-green algae (cyanobacteria), particularly Gloeotrichia, appeared to bear prime responsibility for nitrogen fixation, but photosynthetic bacteria of the genus Rhodopseudomonas were isolated from M. spicatum and shown to support high rates of acetylene reduction.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biebl H., Drews G. Das in-vivo-Spektrum als taxonomisches Merkmal bei Untersuchungen zur Verbreitung von Athiorhodaceae. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1969;123(4):425–452. [PubMed] [Google Scholar]
- Carpenter E. J. Nitrogen fixation by a blue-green epiphyte on pelagic sargassum. Science. 1972 Dec 15;178(4066):1207–1209. doi: 10.1126/science.178.4066.1207. [DOI] [PubMed] [Google Scholar]
- Fay P. Factors influencing dark nitrogen fixation in a blue-green alga. Appl Environ Microbiol. 1976 Mar;31(3):376–379. doi: 10.1128/aem.31.3.376-379.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. Uptake of C4 dicarboxylates and pyruvate by Rhodopseudomonas spheroides. J Bacteriol. 1975 Aug;123(2):471–480. doi: 10.1128/jb.123.2.471-480.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotto J. W., Taylor B. F. N(2) Fixation Associated with Decaying Leaves of the Red Mangrove (Rhizophora mangle). Appl Environ Microbiol. 1976 May;31(5):781–783. doi: 10.1128/aem.31.5.781-783.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINO S., WILSON P. W. Nitrogen fixation by a facultative bacillus. J Bacteriol. 1958 Apr;75(4):403–408. doi: 10.1128/jb.75.4.403-408.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex M., Stewart W. D. Algal nitrogenase, reductant pools and photosystem I activity. Biochim Biophys Acta. 1973 Feb 22;292(2):436–443. doi: 10.1016/0005-2728(73)90049-2. [DOI] [PubMed] [Google Scholar]
- Marsho T. V., Burchard R. P., Fleming R. Nitrogen fixation in the Rhode River estuary of Chesapeake Bay. Can J Microbiol. 1975 Sep;21(9):1348–1356. doi: 10.1139/m75-202. [DOI] [PubMed] [Google Scholar]
- Peterson R. B., Burris R. H. Conversion of acetylene reduction rates to nitrogen fixation rates in natural populations of blue-green algae. Anal Biochem. 1976 Jun;73(2):404–410. doi: 10.1016/0003-2697(76)90187-1. [DOI] [PubMed] [Google Scholar]
- Stewart W. D., Fitzgerald G. P., Burris R. H. In situ studies on N2 fixation using the acetylene reduction technique. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2071–2078. doi: 10.1073/pnas.58.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Leibson P. J., Musil R. J., Huang C. Y. Diurnal variation in algal acetylene reduction (nitrogen fixation) in situ. Plant Physiol. 1975 Feb;55(2):273–276. doi: 10.1104/pp.55.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner D., Evans H. J., Seidler R. J. Facultatively anaerobic nitrogen-fixing bacteria from the marine environment. Can J Microbiol. 1974 Jan;20(1):59–64. doi: 10.1139/m74-010. [DOI] [PubMed] [Google Scholar]


