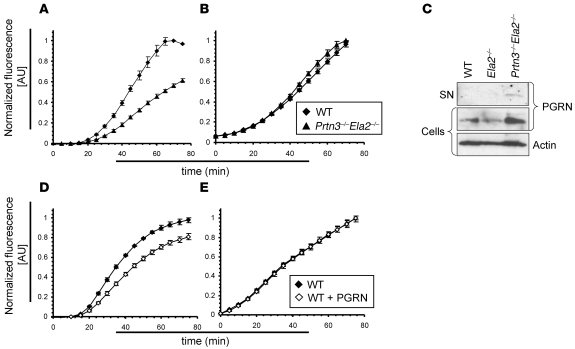
Figure 4. Impaired oxidative burst and PGRN degradation by IC-activated Prtn3–/–Ela2–/– neutrophils.
Oxidative burst as the readout for neutrophil activation by ICs was measured over time. (A) While no difference was observed during the initial 20-min lag phase of the oxidative burst, Prtn3–/–Ela2–/– neutrophils exhibited diminished ROS production over time compared with WT neutrophils. (B) Bypassing receptor-mediated activation using 25 nM PMA restored the diminished oxidative burst of Prtn3–/–Ela2–/– neutrophils. Results are presented as normalized fluorescence in AU (relative to maximum fluorescence produced by WT cells). Data (mean ± SD) are representative of 3 independent experiments each conducted in triplicate. (C) Isolated mouse neutrophils were activated by ICs in vitro and tested for PGRN degradation by IB. In the cellular fraction, the PGRN (~80 kDa) signal was markedly increased in Prtn3–/–Ela2–/– cells compared with WT and Ela2–/– neutrophils. Intact PGRN was present in the supernatant (SN) of IC-activated Prtn3–/–Ela2–/– neutrophils only, not of WT or Ela2–/– cells. (D and E) Exogenous administration of 100 nM PGRN significantly reduced ROS production of neutrophils activated by ICs (D), but not when activated by PMA (E). Data (mean ± SD) are representative of 3 independent experiments each conducted in triplicate.