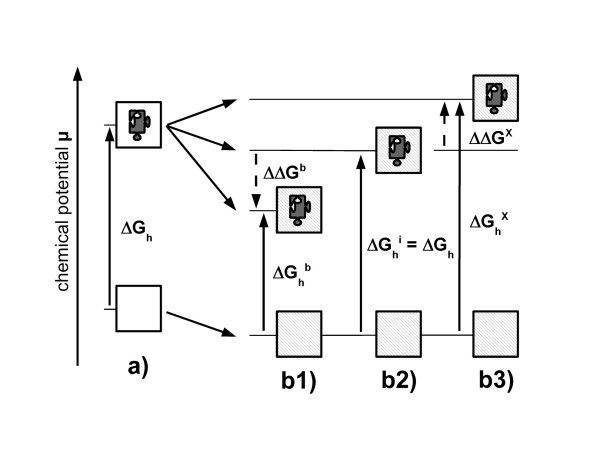
Figure 3.
Thermodynamics of preferential exclusion. In water a) upon addition of a soluble protein the chemical potential μ is raised by the hydration energy ΔGh. A solution of solutes in water b) in general has a lower chemical potential. Depending on whether a solute preferredly binds b1) is inert b2) or is preferredly excluded hydration energies are lower (ΔGhb) equal (ΔGhi) or higher (ΔGhx) than ΔGh. Thus, in a theoretical experiment of transferring a protein from water into a solute solution we either gain energy ΔΔGb, have no energetic effect or have to put energy into the system ΔΔGx.