SYNOPSIS
Objective.
This study assessed the long-term economic implications of a national program to vaccinate all adults treated at sexually transmitted disease (STD) clinics in a single year.
Methods.
A model was developed to track the long-term disease outcomes and costs among a hypothetical cohort of 2 million STD clinic clients accessing services in one year, using data from published sources and demonstration projects at STD clinics in San Diego (California), Illinois, and Denver (Colorado). The model estimated net economic benefits of a routine hepatitis B vaccination policy at STD clinics nationwide compared with no vaccination.
Results.
Without a vaccination program, an estimated 237,021 new hepatitis B virus (HBV) infections would occur over the lifetimes of the 2 million STD clinic clients seen in a single year. HBV-related medical costs and productivity losses would be $1.6 billion. In a national program for routine vaccination at STD clinics, 1.3 million adults would be expected to receive at least one vaccine dose, and an estimated 45% of the new HBV infections expected without vaccination would be prevented. The vaccination program would cost $138 million, HBV infections occurring despite the program would cost $878 million, and clients' time and travel would cost $45 million. The net economic benefit (savings) of routine vaccination would be $526 million. If the indirect costs of lost productivity due to HBV infection are not considered, routine vaccination would have a net cost of $28 million.
Conclusions.
Estimates from this model suggest a national program for routine hepatitis B vaccination of adults at STD clinics would be a cost saving to society.
In the U.S., a majority of adult hepatitis B virus (HBV) infections occur through sexual contact among men who have sex with men, heterosexual people with multiple sexual partners, and through the use of contaminated equipment among injection drug users.1,2 Since 1982, hepatitis B vaccination has been recommended for these high-risk groups, but vaccination coverage among them has been low.3,4 To overcome barriers to vaccination, the Advisory Committee on Immunization Practices (ACIP) has endorsed a strategy that includes routine vaccination of all adults at certain public-health venues where most clients are at high risk for HBV infection, including sexually transmitted disease (STD) clinics, human immunodeficiency virus counseling and testing sites, drug-abuse treatment facilities, and correctional facilities.5 Pilot programs in STD clinics demonstrated that hepatitis B vaccination can be feasibly integrated with other STD prevention services when vaccine was available and provided free of charge to clinic clients.6–9
Publicly funded STD programs operate clinic services for an estimated 1.8 million to 2.2 million individual clients annually (unpublished data, Centers for Disease Control and Prevention).9 Clinics in these programs have the physical infrastructure needed to provide hepatitis B vaccination, but most would require additional funds for vaccine purchase, vaccine administration, staff training, and vaccination record-keeping for a comprehensive vaccination program.7 The costs and benefits of financing such a program have not been adequately addressed, and could be key information for policy makers considering allocating funds for vaccination in these settings. In this study, we estimated the net economic benefits of a hepatitis B vaccination program at public STD clinics nationwide.
METHODS
Study design
We assumed a national program that would offer hepatitis B vaccine to a cohort of 2 million adult clients at STD clinics in one year and tracked the long-term disease outcomes and costs in a decision model (Figure 1). The model compared two scenarios: (1) no hepatitis B vaccination and (2) universal hepatitis B vaccination. In scenario 1, without vaccination, adults who did not have immunity from prior (resolved) infection or vaccination faced the risk of infection and a fraction of them became infected with HBV over their lifetimes. In scenario 2, vaccination was offered to all adults who did not report prior vaccination. Among adults who received vaccination and did not have immunity from prior infection or vaccination, immunity developed based on the number of doses received and estimated vaccine efficacy after each dose. Adults who did not develop immunity from vaccination, or did not have immunity from prior infection, faced the risk of infection, and a fraction of them became infected. Routine vaccination was expected to lower infections in the client population and reduce cost of illness but add program costs. The net economic benefit of routine vaccination was estimated as the difference in expected societal costs under the two scenarios, discounted to 2005 dollars.10 Discounting accounts for differential timing of costs under the two scenarios.11
Figure 1.
Hepatitis B vaccination at STD clinics: decision and Markov models
aBox with solid borders represents an annual health state, arrows indicate allowed transitions; death from each state not shown separately. Box with dashed borders represents health state only allowed in the first year of the model.
bDecompensated cirrhosis includes ascites, variceal hemorrhage, and encephalopathy.
STD = sexually transmitted disease
HBV = hepatitis B virus
Disease progression and risk of HBV infection.
Under both scenarios, people with newly acquired HBV infection were followed in a Markov model of natural history of HBV infection (Figure 1).12 We assumed that the majority of adults with a new HBV infection would remain asymptomatic; about 30% would have acute illness that may include jaundice, hospitalization, and fulminant liver failure (FLF); and 6% would develop chronic infection.13–15 Of those who developed chronic HBV infection, we assumed the majority would remain asymptomatic, 0.06% would be hospitalized annually for acute exacerbation,16 and 0.5% would develop compensated cirrhosis or hepatocellular carcinoma (HCC) each year.17–26 After each year, people with compensated cirrhosis would remain in the same health state or progress. Those whose disease progressed would develop either decompensated cirrhosis or HCC. Patients with FLF, cirrhosis, or HCC would have higher mortality rates compared with the general population. Patients with FLF, decompensated cirrhosis, and HCC were also candidates to receive liver transplantation. Those without infection, with resolved infection, and with chronic hepatitis B but no disease manifestations were assumed to have the same mortality rate as the general population.27 Any of the transitions were permissible to a patient only once during follow-up. We used this model to estimate the long-term outcomes of HBV infection and their costs.
Based on a catalytic model of age-specific prevalence of antibody to hepatitis B-core antigen (anti-HBc) among 300 clients at an STD clinic in San Diego, we estimated the average risk of HBV infection during the remaining lifetime of clinic clients to be about 15%.13,28 Prevaccination testing was not evaluated, as studies had already shown that in populations with HBV infection prevalence lower than 30%, routine vaccination without prevaccination testing was more cost-effective.29–32
Routine vaccination at STD clinics.
We assumed a three-dose series of monovalent adult hepatitis B vaccine administered following the recommended schedule would be offered in one year to all clients who did not report prior vaccination.29 Protective immunity would develop among 90% who completed the series, 75% who received two doses, and 40% who received a single dose.33–35 We assumed vaccine protection would be lifelong and adverse effects would be negligible.
Based on self-reported prior vaccination, we assumed 90% of adults would be eligible for vaccination, and based on the vaccine acceptance in demonstration projects (Viral Hepatitis Integration Projects, VHIPs) in San Diego, Denver, and Illinois, we assumed 50% to 75% would receive at least one dose of vaccine. Among those who received one dose, 40% to 55% would receive a second dose, and 20% to 30% would complete the series.6–8,36 Among those who would be offered vaccine, we assumed 16% would be immune from prior infection (based on anti-HBc positivity).28 We assumed that 10% of vaccine would be wasted due to storage and handling.37
Cost of illness.
Lifetime medical cost and productivity loss from HBV infection was estimated from outputs of the Markov model. The model used updated medical cost data for acute and chronic hepatitis B adjusted for inflation (Table 1). Productivity losses from hepatitis B-related excess mortality, adjusted for unemployment rate and inflation, were estimated from median daily wages of the U.S. population.38 All costs were discounted at a 3% annual rate, a standard rate established by the U.S. Panel on Cost-Effectiveness in Health and Medicine.11
Table 1.
Base-case parameter estimates of vaccination, and HBV infection and disease, among people treated at STD clinics at age 25
In San Diego, California, 10% of the clients reported prior vaccination or infection; we assumed that only half of them would have prior immunity.
Based on data from Sentinel Counties' Surveillance for acute viral hepatitis, Division of Viral Hepatitis, Centers for Disease Control and Prevention, 2000–2003.
Rate of liver transplantation among FLF was the mean ratio of the estimated number of liver transplants for hepatitis B-related acute FLF cases among adults provided by United Network for Organ Sharing to the estimated number of hepatitis B-related acute FLF cases among adults from surveillance data.
Based on the National Inpatient Sample, Healthcare Utilization Project. Agency for Healthcare Research and Quality, Department of Health and Human Services (US), 2003.
Annual progression rate from decompensated cirrhosis to HCC was the same as the annual rate of HCC from compensated cirrhosis.
Annual rates of liver transplantation among decompensated cirrhosis patients were assumed to be half the rates of liver transplantation for HCC.
Estimated ratios of age-specific liver transplantation for HCC from United Network for Organ Sharing, to annual age-specific U.S. HCC incidence from National Program of Cancer Registries, Centers for Disease Control and Prevention, and Surveillance Epidemiology and End Results, National Cancer Institute for the years 1998–2001 (Personal communication, F. Ahmed, National Center for Chronic Disease Prevention and Health Promotion, November 2005).
Mortality rate among HCC patients was estimated from mean annual survival data for patients with liver and intrahepatic bile duct cancer, 1997–2001.
Based on the survival rates of liver transplant for malignant neoplasm, United Network for Organ Sharing as of August 19, 2005.
Estimated by applying Medicare mean national cost to charge ratios to the mean hospitalization charges culled from the National Inpatient Sample, Healthcare Utilization Project, Agency for Healthcare Research and Quality, Department of Health and Human Services (US), 2003.
Annual productivity loss was estimated by multiplying the daily loss by 260 annual workdays times 0.95 for 5% unemployment adjustment. No productivity loss was included for adults >65 years of age.
Vaccine price includes $0.75 per dose Federal Excise Tax that covers compensation for vaccine-related injuries.
The estimated vaccine administration cost was the mean cost from 10,048 records of private sector reimbursement for one dose of adult hepatitis vaccination, estimated from MarketScan® less average private sector vaccine price. This is higher than the administration cost estimated from STD clinics' operations data, and closer to the true cost of vaccine administration.
Transportation cost based on mean per-mile cost of $0.40 and mean round-trip travel of 15 miles. Time cost calculated at $8.50 per hour; mean time spent in preparation, transit, and clinic visit is four hours.
HBV = hepatitis B virus
STD = sexually transmitted disease
FLF = fulminant liver failure
HCC = hepatocellular carcinoma
Program costs.
We assumed vaccines would be offered at no charge to clients; the program would purchase vaccine at the U.S. federal contract price (Table 1);39 and the vaccine administration costs would be similar to those incurred in the VHIPs.6–8,36 Also based on the VHIPs, we included infrastructure costs associated with staff training, supervision, protocol development, and record-keeping associated with vaccination, but excluded the physical infrastructure cost associated with establishing and maintaining clinic facilities.7 All program costs were assumed to occur in the initial year, and, hence, were not discounted. We also included clients' cost of time and travel for the second and third doses of vaccine. We assumed patients would spend a mean of one hour at the clinic at each vaccination visit—the typical time recorded for visits in the San Diego pilot study.28 We also assumed that preparation for a clinic visit would take one hour, and another hour would be spent traveling each way, to and from the clinic. We valued patient time at an average $8.50 per hour, for a total of $34.00 per clinic visit. To estimate the mean cost of patients' time, we assumed 30% of patients at STD clinics are competitive employees, earning $16.13 per hour as estimated from the national monthly data of private average hourly earnings of production workers,40 while 70% earn the minimum wage ($5.15 per hour in 2005).
Base-case and sensitivity analyses
In the U.S., more than 80% of the adults attending STD clinics are 30 years of age or younger.9 To reflect this statistic, we ran the base-case analysis for a cohort aged 25 years. For the Markov model, long-term disease progression rates from published studies were converted to annual rates using the formula p=1-exp(-rt); where p is the long-term rate, r is the annual rate, and t is the time between two health states.41 All base-case parameter values are reported in Table 1. We used univariate sensitivity analysis to examine the impact on model outcomes of changes in the uncertain model parameters. We also carried out threshold analyses of the two variables to which model outcomes were most sensitive, to determine the respective input levels at which medical cost savings alone would just compensate for program costs.
RESULTS
Baseline estimation
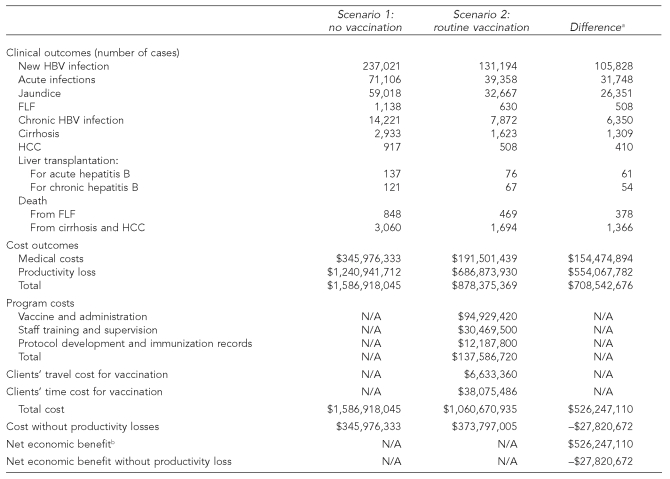
In scenario 1, without a vaccination program, the model estimated 237,021 new HBV infections would occur over the lifetime of the 2 million annual STD clinic clients. These new infections would result in 71,106 acute hepatitis B cases and 14,221 cases of chronic HBV infection. Among people with acute hepatitis B, 1,138 would develop FLF, 137 would require liver transplantation, and 848 would die. Among those with chronic HBV infection 2,933 would develop cirrhosis, 917 would develop HCC, 121 would require liver transplantation, and 3,060 would die. The societal cost of HBV infections would be $1,587 million: $346 million in medical costs and $1,241 million in productivity losses (Table 2).
Table 2.
Hepatitis B vaccination at STD clinics: expected outcomes and economic implications among 2 million clients
Some differences are inexact due to rounding errors.
Net economic benefit is the difference between the two scenarios of the sum of cost of illness, program costs, and clients' travel and time costs for vaccination.
STD = sexually transmitted disease
HBV = hepatitis B virus
FLF = fulminant liver failure
HCC = hepatocellular carcinoma
N/A = not applicable
In the first year of a national vaccination program (scenario 2), a total of 1.3 million clients would receive vaccination: 626,040 clients would receive a single dose, 306,360 clients would receive two doses, and 399,600 clients would receive three doses of vaccine. This level of vaccine coverage would prevent 105,828 of the new HBV infections expected without vaccination, a 45% reduction. Reduction in infection would avert 31,748 acute hepatitis B cases and resultant complications, including 508 FLF cases, 61 liver transplantations, and 378 deaths. In addition, 6,350 chronic HBV infections would be prevented, which would avert 1,309 cirrhosis cases, 410 HCC cases, 54 liver transplantations, and 1,366 deaths.
The first year of the vaccination program would require 2,708,400 doses of vaccine (including an estimated 10% wastage) and cost $138 million, including $95 million for vaccine and administration, $30.5 million for staff training and supervision, and $12.2 million for protocol development and vaccination record-keeping. In addition, clients' travel and time for vaccination would cost $45 million. HBV infections that occur despite the vaccination program would cost $879 million, including $192 million in medical costs and $687 million in productivity losses. The total cost of HBV infection and management with a vaccination program is $1,061 million (compared with $1,587 million without vaccination), yielding a net economic benefit of $526 million. If indirect costs of potential productivity losses were excluded from the analysis, the vaccination program would have a net cost of $28 million, or $263 per new HBV infection averted.
Sensitivity analysis
The net economic benefit was most sensitive to changes in the cohort age, risk of infection, proportion of clients receiving at least one dose, and mean daily wage—changing 11% to 17%, with a 10% change from base-case value of any of the parameters. Net economic benefit was relatively less sensitive to changes in vaccine efficacy, rates of receiving dose two and dose three among patients receiving dose one, prevalence of immunity from prior infection (anti-HBc prevalence), proportion of new infections with acute hepatitis B, lifetime risk of chronic HBV infection, annual rate of compensated cirrhosis among people with chronic HBV infection, and the annual discount rate; changing 1% to 9%, with a 10% change in each parameter (Figure 2). Net economic benefit changed less than 1%, with a 10% change in each of the other parameters.
Figure 2.
Sensitivity analyses: change in net economic benefit in response to 10% change in model parameter values from base casea
aParameter change is an increment unless otherwise indicated.
Anti-HBc = antibody to hepatitis B core antigen
The net economic benefit without productivity losses was relatively more sensitive to all parameter values, with the lifetime risk of HBV infection, and the proportion of STD clients who accept the first dose of vaccine, as the most influential inputs. In a scenario in which lost productivity due to illness was not considered, the net economic benefits increased by 56%, with a 10% increase of either parameter from baseline levels. We determined that medical cost savings alone would be sufficient to compensate for program-related costs if either the lifetime risk of infection among susceptible patients increased by 3% (to 17.7%) or the proportion of STD clinic clients who accept the first dose of vaccine increased by 13% (to 87.3%).
DISCUSSION
Enhanced disease surveillance in four U.S. counties indicated that 36% of those reported with acute hepatitis B between 1996 and 1998 had been previously treated for an STD.2 This finding provides a strong rationale for vaccinating adults who are provided with health care at STD clinics. Integrating routine hepatitis B vaccination in public STD clinics would require developing a program that provides vaccine, pays for vaccine administration, and trains professionals to administer vaccination. Our estimates indicate that such an undertaking would be economically beneficial to the country. Expenditures on vaccination would decrease long-term disease incidence and avert costs, with a net savings to society of more than $500 million in the program's first year.
Despite the anticipated economic benefit, funding for routine vaccination for adults who are un- or underinsured has been inadequate. Over the past decade, in the absence of a national program, state and local STD programs have developed hepatitis B vaccination programs and sought financing from existing sources with limited success. A 2001 survey of STD programs found that 33% of programs had a policy for hepatitis B vaccination and about 65% of clinics in these program areas offered vaccine.42 However, only 26% offered vaccine to all STD clinic clients, and the lack of vaccine funding was cited as the major barrier to program implementation.43 Thus, STD programs nationwide require substantial additional financing to implement ACIP recommendations for adult hepatitis B vaccination.7
Limitations
Our analysis has several limitations. First, the model assumes that scale-up vaccination for the target population in one year is feasible. Although the model estimates were based on pilot programs, if program activities could not be scaled up during this time frame, our model may overestimate the benefits of a vaccination program. Second, we expect that sufficient funds would be available in a national program to achieve the level of vaccine compliance assumed in the base case; if lower vaccine completion rates were allowed, both costs and net savings would be lower.44 Our estimated cost of illnesses was conservative because we included productivity loss from mortality only, and excluded antiviral treatment costs for chronic hepatitis B. Both acute and chronic hepatitis B-related illnesses could lead to a patient's inability to work at least in the short term and increase the cost of illness. Also, antiviral treatment costs are likely to increase the costs of illness.45 With higher costs of illness, the net economic benefit of vaccination would be higher. In the absence of a more exact measurement, we also assumed a constant rate of development of both HCC and cirrhosis, likely overestimating the cost of disease by increasing the burden at younger ages. Finally, we did not consider the potential herd-immunity effects of routine vaccination within or outside the cohort, which is likely to make vaccination more cost-effective.46
CONCLUSION
In the U.S., programs providing routine infant vaccination since 1991 and catch-up adolescent vaccination since 1995 have led to dramatic declines in hepatitis B incidence.1,5,47 Currently, more than 90% of new infections occur among adults, and many of those infected have previously sought health care in venues such as STD clinics, HIV counseling and testing sites, drug abuse treatment facilities, and correctional facilities.1,2,5 National programs that would finance hepatitis B vaccination in these settings can improve vaccination coverage among people at risk for HBV infection, and reduce the burden of hepatitis B among adults in the U.S. As highly immunized cohorts age, vaccinating adults will become a less essential strategy; however, implementing these programs can accelerate elimination of HBV infection among adults until highly immunized cohorts vaccinated through routine infant and adolescent vaccination programs reach adulthood.
Footnotes
This research was supported in part by the Centers for Disease Control and Prevention (CDC) Cooperative Agreements: U50CCU/519083, U50CCU/819041, U50CCU/919053.
The findings and conclusions in this article are those of the authors and do not necessarily reflect the views of CDC.
REFERENCES
- 1.Wasley A, Miller JT, Finelli L. Surveillance for acute viral hepatitis—United States, 2005. MMWR Surveill Summ. 2007;56(3):1–24. [PubMed] [Google Scholar]
- 2.Goldstein ST, Alter MJ, Williams IT, Moyer LA, Judson FN, Mottram K, et al. Incidence and risk factors for acute hepatitis B in the United States, 1982–1998: implications for vaccination programs. J Infect Dis. 2002;185:713–9. doi: 10.1086/339192. [DOI] [PubMed] [Google Scholar]
- 3.Recommendation of the Advisory Committee on Immunization Practices (ACIP): inactivated hepatitis B virus vaccine. MMWR Morb Mortal Wkly Rep. 1982;31(24):317–22. 327-8. [PubMed] [Google Scholar]
- 4.Jain N, Yusuf H, Wortley PM, Euler GL, Walton S, Stokley S. Factors associated with receiving hepatitis B vaccination among high-risk adults in the United States: an analysis of the National Health Interview Survey, 2000. Fam Med. 2004;36:480–6. [PubMed] [Google Scholar]
- 5.Mast EE, Weinbaum CM, Fiore E, Alter MJ, Bell BP, Finelli L, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part II: immunization of adults. MMWR Recomm Rep. 2006;55(RR-16):1–33. [PubMed] [Google Scholar]
- 6.Hepatitis B vaccination among high-risk adolescents and adults—San Diego, California, 1998–2001. MMWR Morb Mortal Wkly Rep. 2002;51(28):618–21. [PubMed] [Google Scholar]
- 7.Harris JL, Jones TS, Buffington J. Hepatitis B vaccination in six STD clinics in the United States committed to integrating viral hepatitis prevention services. Public Health Rep. 2007;122(Suppl 2):42–7. doi: 10.1177/00333549071220S208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subiadur J, Harris JL, Rietmeijer CA. Integrating viral hepatitis prevention services into an urban STD clinic: Denver, Colorado. Public Health Rep. 2007;122(Suppl 2):12–7. doi: 10.1177/00333549071220S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landry DJ, Forrest JD. Public health departments providing sexually transmitted disease services. Fam Plann Perspect. 1996;28:261–6. [PubMed] [Google Scholar]
- 10.Messonnier M, Meltzer M. Cost-benefit analysis. In: Haddix AC, Teutsch SM, Corso PS, editors. Prevention effectiveness: a guide to decision analysis and economic evaluation. 2nd ed. New York: Oxford University Press; 2003. pp. 127–55. [Google Scholar]
- 11.Corso PS, Haddix AC. Time effects. In: Haddix AC, Teutsch SM, Corso PS, editors. Prevention effectiveness: a guide to decision analysis and economic evaluation. 2nd ed. New York: Oxford University Press; 2002. pp. 92–102. [Google Scholar]
- 12.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decision Making. 1993;13:322–38. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 13.Coleman PJ, McQuillan GM, Moyer LA, Lambert SB, Margolis HS. Incidence of hepatitis B virus infection in the United States, 1976–1994: estimates from the National Health and Nutrition Examination Surveys. J Infect Dis. 1998;178:954–9. doi: 10.1086/515696. [DOI] [PubMed] [Google Scholar]
- 14.McMahon BJ, Alward WL, Hall DB, Heyward WL, Bender TR, Francis DP, et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985;151:599–603. doi: 10.1093/infdis/151.4.599. [DOI] [PubMed] [Google Scholar]
- 15.Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci. 1993;253:197–201. doi: 10.1098/rspb.1993.0102. [DOI] [PubMed] [Google Scholar]
- 16.Department of Health and Human Services (US) National inpatient sample, Healthcare Cost and Utilization Project. 2003. [cited 2005 Aug 26]. Available from: URL: http://hcupnet.ahrq.gov.
- 17.Fattovich G, Brollo L, Giustina G, Noventa F, Pontisso P, Alberti A, et al. Natural history and prognostic factors for chronic hepatitis type B. Gut. 1991;32:294–8. doi: 10.1136/gut.32.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fattovich G, Rugge M, Brollo L, Pontisso P, Noventa F, Guido M, et al. Clinical, virologic and histologic outcome following seroconversion from HBeAg to anti-HBe in chronic hepatitis type B. Hepatology. 1986;6:167–72. doi: 10.1002/hep.1840060203. [DOI] [PubMed] [Google Scholar]
- 19.Dragosics B, Ferenci P, Hitchman E, Denk H. Long-term follow-up study of asymptomatic HBsAg-positive voluntary blood donors in Austria: a clinical and histologic evaluation of 242 cases. Hepatology. 1987;7:302–6. doi: 10.1002/hep.1840070215. [DOI] [PubMed] [Google Scholar]
- 20.Moreno-Otero R, Garcia-Monzon G, Garcia-Sanchez A, Garcia Buey L, Pajares JM, Di Bisceglie AM. Development of cirrhosis after chronic type B hepatitis: a clinicopathologic and follow-up study of 46 HBeAg-positive asymptotic patients. Am J Gastroenterol. 1991;86:560–4. [PubMed] [Google Scholar]
- 21.Sampliner RE, Hamilton FA, Iseri OA, Tabor E, Boitnott J. The liver histology and frequency of clearance of the hepatitis B surface antigen (HBsAg) in chronic carriers. Am J Med Sci. 1979;227:17–22. doi: 10.1097/00000441-197901000-00002. [DOI] [PubMed] [Google Scholar]
- 22.McMahon BJ, Holck P, Bulkow L, Snowball M. Serologic and clinical outcomes of 1536 Alaska Natives chronically infected with hepatitis B virus. Ann Intern Med. 2001;135:759–68. doi: 10.7326/0003-4819-135-9-200111060-00006. [DOI] [PubMed] [Google Scholar]
- 23.Alward WL, McMahon BJ, Hall DB, Heyward WL, Francis DP, Bender TR. The long-term serological course of asymptomatic hepatitis B virus carriers and the development of primary hepatocellular carcinoma. J Infect Dis. 1985;151:604–9. doi: 10.1093/infdis/151.4.604. [DOI] [PubMed] [Google Scholar]
- 24.Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168–74. doi: 10.1056/NEJMoa013215. [DOI] [PubMed] [Google Scholar]
- 25.Beasley RP, Hwang L-Y. Overview on the epidemiology of hepatocellular arcinoma. In: Hollinger FB, Lemon SB, Margolis HS, editors. Viral hepatitis and liver disease. Baltimore: Williams & Wilkins; 1991. pp. 532–5. [Google Scholar]
- 26.Sherman M, Peltekian KM, Lee C. Screening for hepatocellular in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432–8. [PubMed] [Google Scholar]
- 27.Arias E. United States life tables, 2002. Natl Vital Stat Rep. 2004 Nov 10;53:1–38. [PubMed] [Google Scholar]
- 28.Gunn RA, Murray PJ, Ackers ML, Hardison WG, Margolis HS. Screening for chronic hepatitis B and C virus infections in an urban sexually transmitted disease clinic: rationale for integrating services. Sex Transm Dis. 2001;28:166–70. doi: 10.1097/00007435-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Sexually transmitted diseases treatment guidelines 2002. MMWR Recomm Rep. 2002;51(RR-6):1–78. [PubMed] [Google Scholar]
- 30.Kim SY, Billah K, Lieu TA, Weinstein MC. Cost effectiveness of hepatitis B vaccination at HIV counseling and testing sites. Am J Prev Med. 2006;30:498–506. doi: 10.1016/j.amepre.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Hepatitis B vaccination of inmates in correctional facilities—Texas, 2000–2002. MMWR Morb Mortal Wkly Rep. 2004;53(30):681–3. [PubMed] [Google Scholar]
- 32.Blostein J, Clark PA. Cost-effectiveness of preimmunization hepatitis B screening in high-risk adolescents. Public Health Rep. 2001;116:165–8. doi: 10.1093/phr/116.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andre FE. Summary of safety and efficacy data on a yeast-derived hepatitis B vaccine. Am J Med. 1989;87(3A):14S–20S. doi: 10.1016/0002-9343(89)90525-1. [DOI] [PubMed] [Google Scholar]
- 34.Jilg W, Deinhardt F. Results of immunization with a recombinant yeast-derived hepatitis B vaccine. J Infect. 1986;13(Suppl A):47–51. doi: 10.1016/s0163-4453(86)92683-6. [DOI] [PubMed] [Google Scholar]
- 35.Hadler SC, Francis DP, Maynard JE, Thompson SE, Judson FN, Echenberg DF, et al. Long-term immunogenicity and efficacy of hepatitis B vaccine in homosexual men. N Engl J Med. 1986;315:209–14. doi: 10.1056/NEJM198607243150401. [DOI] [PubMed] [Google Scholar]
- 36.Zimmerman R, Finley C, Rabins C, McMahon K. Integrating viral hepatitis prevention into the STD prevention program in Illinois (excluding Chicago), 1999–2005. Public Health Rep. 2007;122(Suppl 2):18–23. doi: 10.1177/00333549071220S204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller MA, Redd S, Hadler S, Hinman A. A model to estimate the potential economic benefits of measles eradication for the United States. Vaccine. 1998;16:1917–22. doi: 10.1016/s0264-410x(98)00125-x. [DOI] [PubMed] [Google Scholar]
- 38.Bureau of Labor Statistics (US) Usual weekly earnings of wage and salary workers: fourth quarter 2003; table 2: median weekly earnings of full-time wage and salary workers by age, race, Hispanic or Latino ethnicity, and sex, fourth quarter 2003 averages, not seasonally adjusted. press release. 2004. Jan 16, [cited 2004 Nov 8]. Available from: URL: http://www.bls.gov/news.release/archives/wkyeng_01162004.pdf.
- 39.Centers for Disease Control and Prevention (US) Vaccines for Children program: vaccine price list. [cited 2005 Aug 25]. Available from: URL: http://www.cdc.gov/vaccines/programs/vfc/cdc-vac-price-list.htm.
- 40.Bureau of Labor Statistics (US) Average hourly earnings of production and nonsupervisory workers on private nonfarm payrolls by industry sector and selected industry detail seasonally adjusted. [cited 2007 Nov 3]. Available from: URL: http://www.bls.gov/webapps/legacy/cesbtab4.htm.
- 41.Petitti DB. New York: Oxford University Press; 2000. Meta-analysis, decision analysis, and cost-effectiveness analysis: methods for quantitative synthesis in medicine. [Google Scholar]
- 42.Gilbert LK, Bulger J, Scanlon K, Ford K, Bergmire-Sweat D, Weinbaum C. Integrating hepatitis B prevention into sexually transmitted disease services: U.S. sexually transmitted disease program and clinic trends—1997 and 2001. Sex Transm Dis. 2005;32:346–50. doi: 10.1097/01.olq.0000154503.41684.5d. [DOI] [PubMed] [Google Scholar]
- 43.Wilson BC, Moyer L, Schmid G, Mast E, Voigt R, Mahoney F, et al. Hepatitis B vaccination in sexually transmitted disease (STD) clinics: a survey of STD programs. Sex Transm Dis. 2001;28:148–52. doi: 10.1097/00007435-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Billah K, Buffington J, Weinbaum C, Mast E, Subiadur J, Zimmerman R, et al. Economic implications of hepatitis B vaccination of adults at sexually transmitted disease clinics in the United States. Poster abstract in the proceedings of National STD Prevention Conference; 2004 Mar 8–11; Philadelphia. [Google Scholar]
- 45.Kanwal F, Gralnek IM, Martin P, Dulais GS, Farid M, Spiegel BM. Treatment alternatives for chronic hepatitis B virus infection: a cost-effectiveness analysis. Ann Intern Med. 2005;142:821–31. doi: 10.7326/0003-4819-142-10-200505170-00007. [DOI] [PubMed] [Google Scholar]
- 46.Menzies R, McIntyre P. Vaccine preventable diseases and vaccination policy for indigenous populations. Epidemiol Rev. 2006;28:71–80. doi: 10.1093/epirev/mxj005. [DOI] [PubMed] [Google Scholar]
- 47.Hepatitis B vaccination—United States, 1982–2002. MMWR Morb Mortal Wkly Rep. 2002;51(25):549–52. 563. [PubMed] [Google Scholar]