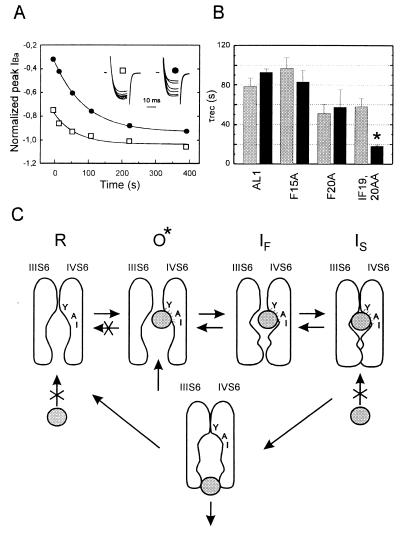
Figure 4.
IBa recovery from slow inactivation and unblock from use-dependent block by 100 μM (−)D600. (A) (□) Time course of the slow component in AL1 IBa recovery from inactivation in control after a 10 sec prepulse to 10 mV (see Methods). (•) Time course of peak IBa recovery from block by 100 μM (−)D600 induced by 15 test pulses (100 ms) applied at 0.1 Hz pulses from −80 mV to 10 mV in oocytes expressing the fast inactivating chimera AL1. The inset shows IBa during 20 ms test pulses to 10 mV applied at different intervals after a single 10 sec prepulse to 10 mV (□) or use-dependent block (•). Peak current values are normalized (see Inset) to IBa of the prepulse (□) or to the first current of the train (•), respectively. Time courses of IBa recovery from use-dependent block and inactivation were fitted to single exponential functions; respective time constants are τunblock = 94 sec and τslow = 68 sec. (B) Mean time constants of IBa recovery from inactivation (shaded) and from use-dependent block (solid) by 100 μM (−)D600 of chimeras AL1, F15A, and F20A. To observe a significant amount of IBa inhibition during a 0.1 Hz train in IF19,20AA we increased the applied (−)D600 concentration to 300 μM. Statistical significant difference in recovery from block and slow inactivation is indicated by asterisks (*P < 0.05). (C) Model for PAA interaction with Ca2+ channels illustrating drug induced slow inactivation. Access of the PAA molecule (symbolized by the grey ball) to the receptor site in the resting state (R) is restricted. After removal of putative pore-orientated guarding amino acids the drug can enter the channel from the cytoplasmic site in the open conformational state (O*) and subsequently interact with its receptor site (24, 25). According to (8) pore-orientated amino acids of transmembrane segment IVS6 contribute high affinity determinants of the PAA receptor site (11, 25). After PAA interaction with its receptor site the channels become non conducting and transitions of Ca2+ channels to the resting state are restricted (see also refs. 27 and 28). If activation and inactivation are coupled processes Ca2+ channels will move to the fast inactivated state (IF) and subsequently accumulate in the slow inactivated state (IS) even if the drug does not directly affect the rate of Ca2+ channel inactivation. Consequently the amount of use-dependent block during a train of pulses will crucially depend on the rate of channel inactivation (see Fig. 2).