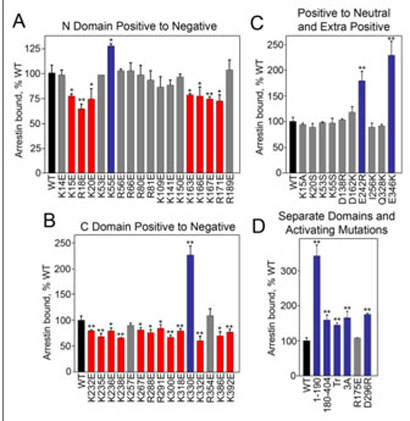
FIGURE 3. Asubset of exposed charges in arrestin is important for microtubule binding.
The indicated in vitro translated radiolabeled arrestins (200 fmol) were incubated with 20 μg of MTs in a total volume of 100 μl for 20 min at 30 °C. The samples were centrifuged and MT-bound arrestin in the pellet was quantified as described in Fig. 1. The effects of positive charge reversal mutations in the arrestin N-domain (A) and C-domain (B) on MT binding are shown. C, charge neutralization mutations do not have the same effect as charge reversal at the same position. The addition of extra positive charges only affects binding in specific locations. D, the separately expressed N-domain-(1–190) and C-domain-(180–404) bind significantly better than full-length arrestin. Mutations that activate arrestin with regard to receptor binding also enhance MT binding, with the exception of R175E. Mutations that significantly decrease, increase, or do not change binding compared with WT are shown in red, blue, and gray, respectively. 3A (F375A, V376A, F377A), Tr (1–378). Means ± S.D. of three experiments are shown. *, p < 0.05; **, p < 0.01, as compared with WT.