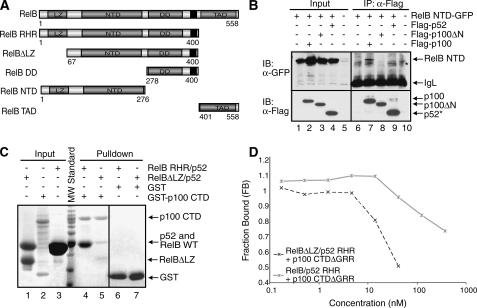
FIGURE 5.
The LZ domain of RelB is involved in p100 binding. A, schematic representations of RelB domains and the deletion mutants of RelB used in the binding experiments. The black regions represent the nuclear localization signals. B, co-IP experiments showing binding interaction between RelB NTD-GFP and wt or deletion mutants of p100 with the FLAG peptide. The cells were cotransfected with RelB NTD-GFP and p100 mutants, extracts were immunoprecipitated by α-FLAG antibody and immunoblotted with FLAG (bottom panel) and GFP (top panel). An asterisk indicates a nonspecific band. C, in vitro GST pulldown experiments were done using equal amounts of pure recombinant proteins showing that the N-terminal LZ domain of RelB is involved in the binding interaction with the CTD of p100. Input and pulldown samples were separated by SDS-PAGE followed by Coomassie staining. Please note that His-RelB RHR and p52 RHR co-migrate in the SDS-PAGE (lanes 3 and 4). At position 64 of RelB, a cryptic thrombin cleavage site is located; therefore the first 64 residues are removed (lanes 1 and 5). D, fluorescence polarization experiments showing the functional role of the LZ domain of RelB in binding the p100 CTDΔGRR. Fluorescence polarization assay was done the same as in Fig. 3F.