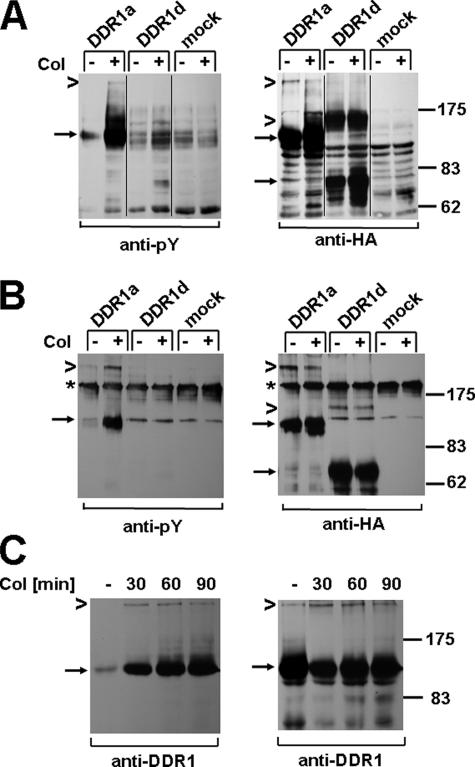
FIGURE 4.
DDR1 dimers are tyrosine-phosphorylated upon collagen stimulation. 293 cells were transfected with DDR1a-HA, DDR1d-HA, or control vector and stimulated with 10 μg/ml type I collagen (Col) for 90 min. A, equal amounts of total cell lysates were resolved by nonreducing gel electrophoresis and Western blots probed with anti-phosphotyrosine (Py) antibody (left panel) and reprobed with anti-HA antibody (right panel). Arrows point to monomeric DDR1a and DDR1d, and chevrons point to dimeric DDR1 forms, respectively. B, cell lysates were subjected to anti-HA immunoprecipitation, and the presence of tyrosine-phosphorylated, dimeric DDR1a was confirmed by anti-phosphotyrosine Western blotting (left panel). The asterisks indicate a nonspecific band, likely IgG oligomers, that showed cross-reactivity under nonreducing conditions. The reprobe of the same blot with anti-HA antibody is shown on the right panel. A set of data representative for two repeats of these experiments is shown.