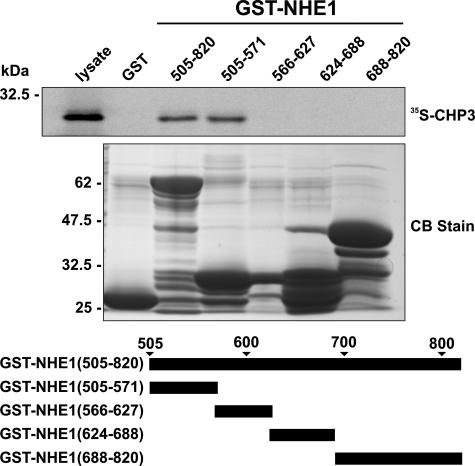
FIGURE 1.
Mapping the CHP3-binding region of NHE1. Protein binding pulldown assays were used to delineate the site of interaction of CHP3 within the cytoplasmic C terminus of NHE1. GST fusion proteins containing full-length (amino acids 505–820) or partial segments spanning the length of the C terminus of NHE1 were generated in E. coli. Purified GST fusion proteins were incubated with 35S-labeled CHP3 protein synthesized in vitro in rabbit reticulocyte lysates using a transcription-translation coupling reaction in the presence of [35S]methionine. Complexes of GST-NHE1 and 35S-labeled CHP3 were isolated from the lysates using glutathione-Sepharose™ beads and subjected to SDS-PAGE, as described under “Experimental Procedures.” The radioactivity was analyzed using a PhosphorImager (upper panel). To verify equivalent gel loading of the GST fusion proteins, a parallel gel was stained with Coomassie Blue (CB) dye (lower panel). Data shown are representative of at least three independent experiments.