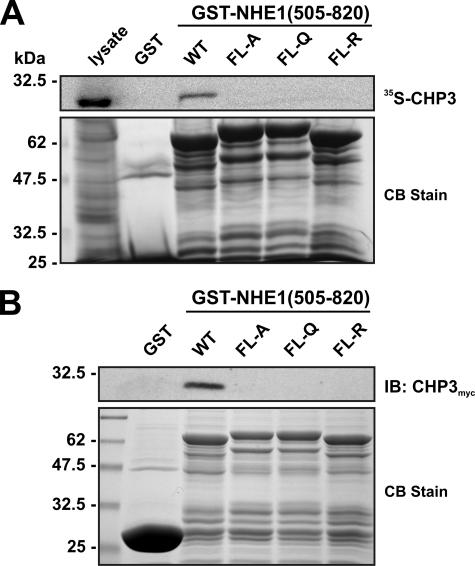
FIGURE 2.
Mutational analysis of the CHP3-binding site of NHE1. Four hydrophobic amino acids within the juxtamembrane region of NHE1 (530FLDHLL535) that were shown previously to be crucial for direct binding of CHP1 and CHP2 were simultaneously mutated in the GST-NHE1(505–820) construct to either Ala, Gln, or Arg (FL-A, FL-Q, or FL-R). Purified wild-type (WT) and mutant GST-NHE1 fusion proteins were incubated with either rabbit reticulocyte lysates containing in vitro synthesized 35S-labeled CHP3 protein (A) or lysates of CHO cells transiently expressing exogenous CHP3myc (B). Complexes of GST-NHE1 and 35S-labeled CHP3 or CHP3myc were isolated using glutathione-Sepharose™ beads and subjected to SDS-PAGE. The levels of 35S-labeled CHP3 were analyzed using a PhosphorImager (A, upper panel), whereas the amounts of CHP3myc were detected by immunoblotting (IB) using a primary mouse monoclonal anti-Myc antibody and a secondary goat anti-mouse antibody conjugated to horseradish peroxidase (B, upper panel). To verify equivalent gel loading of the GST fusion proteins, parallel gels were stained with Coomassie blue (CB) dye (A and B, lower panel). Data shown are representative of at least three independent experiments.