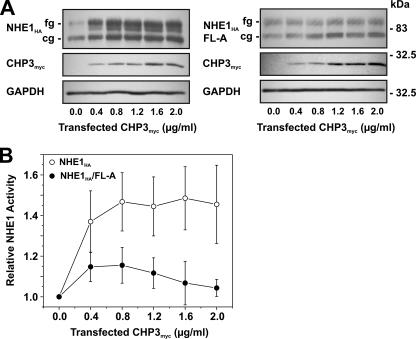
FIGURE 6.
Effect of overexpression of CHP3 on NHE1 abundance and activity. AP-1 cells stably expressing either wild-type NHE1HA or CHP3-binding defective mutant NHE1HA/FL-A were cultured to subconfluence in 6-well plates (10-cm2/well) for immunoblot analyses (A) or 24-well plates (2-cm2/well) for measurement of NHE1 activity (B), and then transiently transfected with empty vector or increasing amounts of the CHP3myc-containing expression plasmid relative to empty vector. The total concentration of the transfected plasmid DNA remained constant at 2 μg of DNA/ml serum-free culture medium (2.5 ml per 10-cm2 dish or 0.5 ml per 2-cm2 dish). A, following transfection (24 h), cell lysates were prepared and analyzed for NHE1HA and CHP3myc expression by SDS-PAGE and immunoblotting. Immunoreactive bands corresponding to the fully glycosylated and core-glycosylated forms of NHE1HA and CHPmyc were detected using a primary mouse monoclonal anti-HA and anti-Myc antibody, respectively, and a secondary goat anti-mouse antibody conjugated to horseradish peroxidase. As a control for protein loading, the blots were stripped and reprobed for expression of endogenous GAPDH using a primary mouse monoclonal anti-GAPDH antibody and a secondary goat anti-mouse antibody conjugated to horseradish peroxidase. B, Na+/H+ exchange activity of cells expressing wild-type NHE1HA and mutant NHE1HA/FL-A were measured as a function of CHP3 abundance. NHE activity was determined as the initial rates of amiloride-inhibitable 22Na+ influx following an acute intracellular acid load induced by prepulsing with NH +4, as described under “Experimental Procedures.” To facilitate comparison, the data were normalized to cells that do not express CHP3. Values represent the mean ± S.E. of three experiments, each performed in triplicate.