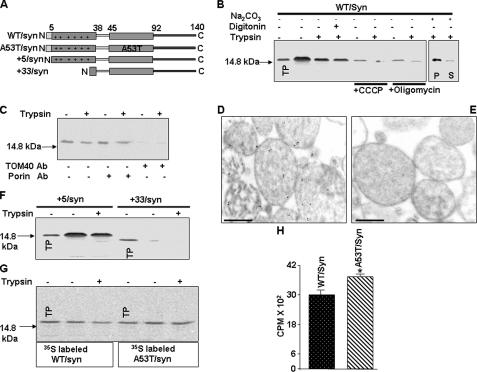
FIGURE 1.
Characterization of mitochondrial targeting properties of α-synuclein by mitochondrial import assay. A, description of wild-type (WT), familial, and N-terminal deletion constructs of α-synuclein. B, in vitro mitochondrial import of 35S-labeled WT/synuclein, in isolated rat liver mitochondria as described under “Materials and Methods” using 200 μg of trypsin/ml of reaction (+). Mitochondria were preincubated with or without added inhibitors (50 μm carbonyl cyanide-m-lorophenylhydrazone (CCCP) or 50 μm oligomycin) at 25 °C for 10 min before initiating the in vitro transport of WT/synuclein. C, effects of antibodies to porin and TOM40 on mitochondrial import of α-synuclein. Mitochondria were preincubated with antibodies (5 μg/ml) to porin and TOM40 at room temperature for 30 min prior to the initiation of import. Immunoelectron microscopy of mitochondrial imported unlabeledα-synuclein using rabbit polyclonal antibodies toα-synuclein (D) and the preabsorbed α-synuclein antibody (E). Sections were stained with secondary antibody conjugated to 10 nm gold. Bar = 500 nm. F, in vitro mitochondrial import of +5/synuclein and +33/synuclein. G and H, effects of A53T mutation on mitochondrial import of α-synuclein (G, autoradiogram and H, quantitation of trypsin-protected α-synuclein bands). Values are mean ± S.D. from three separate experiments. *, p < 0.05 as compared with WT/synuclein (Student's t test). 250 μg of mitochondrial protein were used for electrophoresis. TP = translation product (20% of total input), S = supernatant, P = pellet.