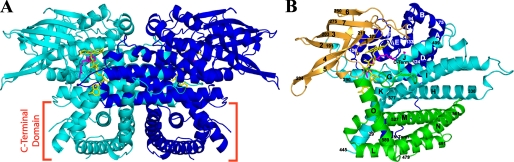
FIGURE 1.
A, ribbon diagram of the overall fold of the human VLCAD dimer. The monomers are represented in cyan and blue. The FADs are shown in yellow, and a partial model of C14-CoA is shown in magenta. The C-terminal domain is marked near the bottom of the figure. B, overall polypeptide fold of a VLCAD monomer showing the N-terminal α-dom1 (blue), the β-sheet domain (gold), α-dom2 (cyan), and the C-terminal α-dom3 (green). The FAD cofactor and the partially hydrolyzed model of substrate/product (C14-CoA) are shown with sticks in yellow and pink, respectively. α-Helices are labeled alphabetically, and β-strands are numbered consecutively from the N to C terminus. The numbers in a smaller font are residue numbers. Unless otherwise noted, all figures were generated using Pymol (52).