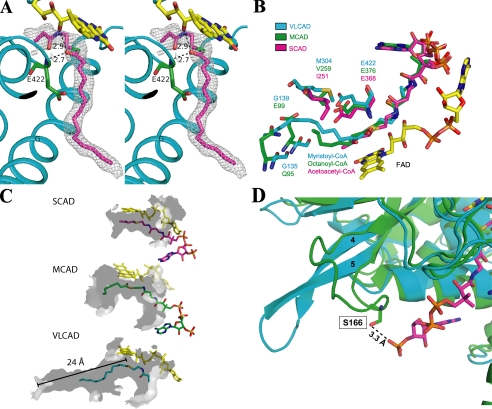
FIGURE 3.
A, stereo diagram of a 2Fo-Fc electron density map (1.0σ) fitted with a partial model of C14-CoA. The catalytic base, Glu-422 (green), is located in a position analogous to the catalytic residues of MCAD and SCAD. As with MCAD and SCAD, substrate position is stabilized by hydrogen bonds between the thioester carbonyl and both the amide nitrogen of the catalytic glutamate (2.7 Å) and the ribityl 2′-hydroxyl of FAD (2.9 Å). Helices are labeled. Part of the pantothenic acid and adenosine pyrophosphate moiety of CoA are not visible. The thioester sulfur is shown as green for clarity. B, overlay of the binding site residues of VLCAD (blue), MCAD (green; ID: 3MDE), and SCAD (magenta; ID: 1JQI). Gln-95 and Glu-99 in MCAD form the bottom of its binding cavity. In VLCAD, the corresponding residues are glycines (Gly-135 and Gly-138). This effectively extends the cavity and allows for much longer substrates to bind. C, surface renderings (inside, light gray; outside, dark gray) of the binding cavities of SCAD, MCAD, and VLCAD illustrate the basis of their substrate chain length specificities. The cavity depths of SCAD, MCAD, and VLCAD, measured from the substrate thioester carbonyls (shown for VLCAD), are 8, 12, and 24 Å. Only a partial model of the CoA moiety of C14-CoA is shown. D, overlay of the substrate binding sites of VLCAD (cyan) and MCAD (green; ID: 3MDE). In MCAD, residue Ser-166 on the loop between β-strands 4 and 5 hydrogen bonds with the 3′-phosphate on the CoA moiety of octanoyl-CoA. In VLCAD, the β-sheet formed by strands 4 and 5 extends much further away from the substrate binding site, thereby precluding an analogous hydrogen bond, and resulting in a wider opening of the substrate binding cavity.