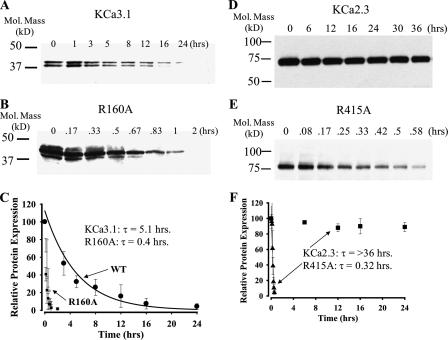
FIGURE 3.
Time course for degradation of wild type and mutant KCa3.1 and KCa2.3 channels. Representative IB showing degradation of wild type KCa3.1 (A), R160A KCa3.1 (B), wild type KCa2.3 (D), and R415 KCa2.3 (E) in the presence of cycloheximide (400 μg/ml) for various periods of time as indicated. For wild type KCa3.1 and KCa2.3, a total of 20 μg of protein was loaded in each lane, whereas 100 μg of total protein was loaded for R160A KCa3.1 and R415A KCa2.3. These time courses for degradation were repeated three times for each construct and the resulting IB digitized, and band intensities for the various time points were determined as a percent change from time 0. The decrease in total protein was fit to an exponential decay function, and the time constant (τ ± S.E.) was determined for each construct. The average intensities and fits are shown in C and F for KCa3.1 and KCa2.3, respectively. Note that the decay of wild type KCa2.3 could not be fit as sufficient protein had not been degraded over a 36-h time period.