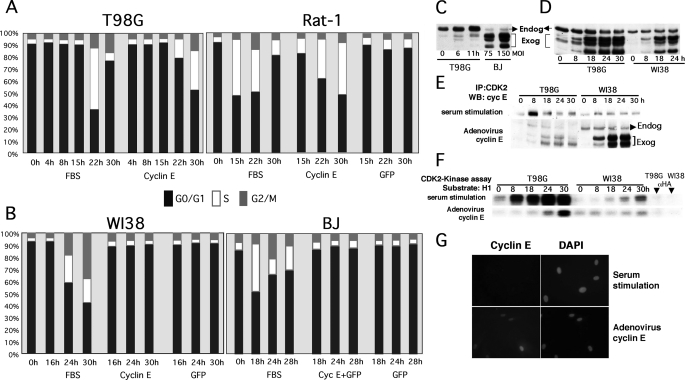
FIGURE 1.
Ectopic expression of cyclin E induces mitogen-independent cell cycle entry in human tumor T98G cells and immortalized diploid rat fibroblasts (Rat-1) but not in NHF, where it fails to activate CDK2 despite proper complex formation and localization. T98G, Rat-1 (A), and human primary fibroblasts (WI38 and BJ) (B) were made quiescent by serum starvation (72 h). Serum-starved cells were transduced at an MOI of 50 (T98G and Rat-1) or 150 (primary fibroblasts) with the indicated recombinant adenoviruses or stimulated with FBS, harvested, and stained with propidium iodide for PI/FACS analysis at the indicated time points. The percentage of cells on each phase of the cell cycle is represented. C, T98G cells were serum-starved and restimulated with FBS and harvested at the indicated time points. Serum-starved BJ cells were transduced with cyclin E adenoviruses at the indicated MOI for 24 h. Note that ectopically expressed cyclin E is resolved as a set of bands with faster mobility than endogenous cyclin E, as previously described (see Ref. 55 and references therein). These forms, which are generated via protease cleavage, bind and activate CDK2 and form complexes with p21/p27. D, T98G and WI38 cells were serum-starved as described in A and transduced with cyclin E adenoviruses or restimulated with 10% FBS. Cells were harvested at the indicated time points. Cyclin E levels were determined by Western blot (WB) analysis. E and F, 250 μg of protein lysate were used to immunoprecipitate (IP) CDK2 complexes with specific antibodies. CDK2 complexes were resolved via SDS-PAGE and probed with cyclin E antibodies (E) or used for in vitro histone H1 kinase assays (F). G, BJ fibroblasts were seeded onto 12-well cluster plates, serum-starved for 72 h, and transduced with the cyclin E adenovirus or stimulated with FBS. After 24 h, cells were fixed, and cyclin E was detected via indirect immunofluorescence. Total DNA was visualized via 4′,6-diamidino-2-phenylindole (DAPI) staining. Cells were visualized under an inverted fluorescence microscope.