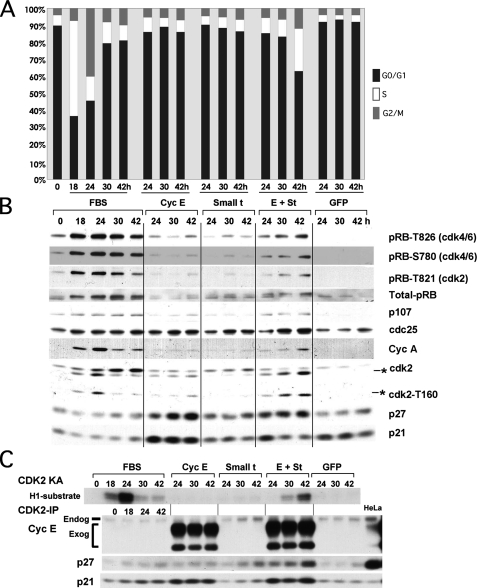
FIGURE 3.
st and cyclin E cooperate to induce phosphorylation of CDK2 on Thr-160, CDK2 activation, pocket protein hyperphosphorylation, E2F-dependent gene expression, and mitogen-independent cell cycle entry without altering the pool of cyclin E-CDK2 complexes bound to p21/p27. BJ fibroblasts were serum-starved as described in the legend to Fig. 1 and transduced with 150 MOI of the indicated adenoviruses or restimulated with FBS. Cells were harvested at the indicated time points and prepared for PI/FACS (A) or Western blot analyses (B). The Thr-160-phosphorylated form of CDK2 is marked with an asterisk. C, protein lysates from the same samples used in B were immunoprecipitated with CDK2 antibodies to determine the composition of CDK2 complexes and measure CDK2-associated kinase activity (KA).