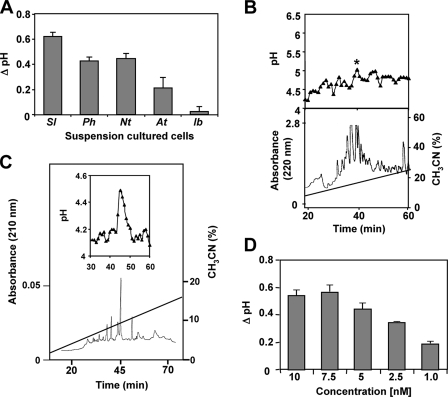
FIGURE 1.
Isolation of IbHypSys. A, alkalinization of the medium of suspension cultured cells of tomato (Sl), petunia (Ph), tobacco (Nt), Arabidopsis (At), and sweet potato (Ib). Ten μl of a crude extract from sweet potato (I. batatas) leaves were added to 1 ml of cells. The change in pH was recorded after 30 min. The data represent the average of three separate experiments. B, lower panel, the crude extract was dissolved in 0.1% trifluoroacetic acid, H2O and chromatographed through a preparative reversed-phase C18-HPLC column with a 90-min gradient from 0 to 40% acetonitrile at a flow rate of 4 ml/min (Stage III of purification). Upper panel, alkalinization assays of 10 μl from each fraction when added to 1 ml of tomato suspension culture as described under “Experimental Procedures.” C, further purification of the peptide eluting in the fraction of the peak in the upper panel of B, marked (*) as described in Stages IV and V of the purification. Lower panel, the peptide mixture was separated on a narrow bore reversed-phase C18 HPLC column with 10 mm potassium phosphate, pH 6, at a flow rate of 0.25 ml/min. A 90-min gradient was performed from 0 to 40% with an elution buffer consisting of 10 mm potassium phosphate in 50% acetonitrile, pH 6. One-minute fractions were monitored at 220 nm (Stage V of purification). D, the concentration dependence of the purified isopeptides from the final purification in Stage V in the alkalinization assay. The change in pH of the suspension cell medium in response to the increasing concentration of peptides was recorded 30 min after the addition of peptide. The change in pH was compared with distilled H2O controls of equal volume (10 μl). The data represent the average of three separate experiments.