Table 1.
Selected examples of a vanadium-catalyzed SOS reaction a
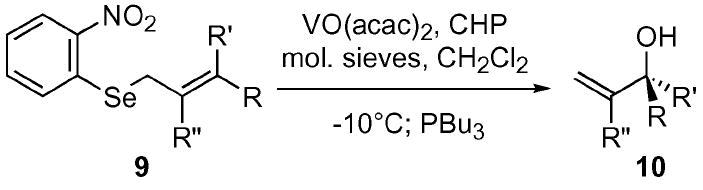
| Entry | R | R′ | R″ | Yield b |
|---|---|---|---|---|
| a c | −C6H13 | H | H | 70% |
| b |
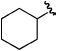
|
H | H | 75% |
| c |
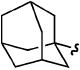
|
H | H | 84% |
| dd |
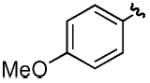
|
H | H | 66% |
| e |
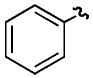
|
H | H | 65% |
| f |
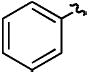
|
H | CH3 | 70% |
| g | H | −C6H13 | H | 89% |
| hd | H |
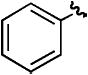
|
H | 86% |
All reactions unless otherwise noted were performed at 0.3 M concentration of substrate using 1.2 eq. tributylphosphine to quench the selenenate intermediate.
All yields are isolated yields after chromatography over silica gel.
This reaction was quenched with triphenyl-phosphine.
t-Butyl hydroperoxide was substituted for cumene hydroperoxide to simplify purification.
