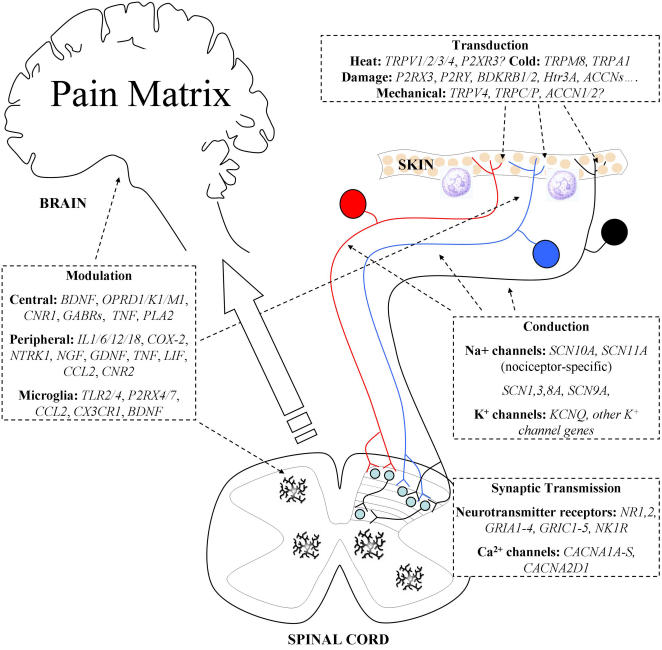
Figure 1. Genes Involved in Pain Perception and Modulation.
Noxious stimuli are detected by primary afferent neurons innervating the skin, muscle, and viscera. The cell bodies of these damage-sensing neurons (nociceptors) are found in dorsal root ganglia, except for neurons innervating craniofacial tissues, which have cell bodies in the trigeminal ganglia. Nociceptors are commonly divided into three groups: peptidergic (blue, NGF-responsive) and nonpeptidergic (black, GDNF-responsive) unmyelinated C-fibres, and myelinated Aδ-fibres (red, NGF-responsive). Gene expression profiles differ between these groups, with functional distinctions (reviewed in [62]). Specialised receptors, expressed in the peripheral termini of these neurons, allow noxious stimuli to be transduced into electrical impulses. While the majority of nociceptors are polymodal (they can detect a variety of noxious stimuli), specific receptors exist for each stimulus modality. The receptors for certain modalities, including mechanical and heat damage, are currently unidentified, although several promising candidates have been proposed. In particular, specific TRP channels appear to be involved in transduction of several stimulus modalities (e.g., [56],[57],[63],[64]). The local membrane depolarisation generated by stimulus transduction is transmitted along the axon by voltage-gated sodium channels, some of which are expressed specifically in nociceptors. The channels NaV1.7 (SCN9A) and NaV1.8 (SCN10A) have both been shown to play an important role in nociceptive transmission [6],[7],[11]. Transmission is modulated by the actions of potassium channels, which generally act to reduce excitability. Regulation of spike frequency by potassium channels has also been reported. The sensitivity of both transduction and action potential transmission by nociceptors can be altered by inflammatory and other mediators, released by immune system cells and damaged neurons. This modulation is an important component of inflammatory hyperalgesia and neuropathic allodynia, in which neuronal sensitivity is greatly increased. Nociceptors terminate in laminae 1 (Aδ-fibres) and 2 (C-fibres) of the spinal cord dorsal horn, forming synapses with nociceptive-specific spinal projection neurons. Some also synapse with wide dynamic range projection neurons in lamina 5, while light touch neurons synapse in deeper laminae. Synaptic transmission occurs through NMDA, AMPA, and kainate receptors, in addition to neuropeptide and proton-mediated transmission. Neurotransmitter release is controlled by voltage-gated Ca2+ channels in the presynaptic membrane. Regulation of synaptic strength is achieved by many mechanisms, including long-term potentiation, whereby repeated activity within a frequency range results in increased response to subsequent inputs. Additionally, microglia resident in the spinal cord respond to damage or inflammation by releasing growth factors and cytokines that alter the excitability of spinal neurons. In certain cases, this can lead to the ongoing activation of pain pathways. Brain Derived Neurotrophic Factor (BDNF), fractalkine, and chemokines have been invoked as important mediators. Descending input from the brain to the spinal cord can both inhibit and facilitate the transmission of information in nociceptive circuits. From the spinal cord, information is transmitted to the brain stem via nociceptor-specific and wide dynamic range projection neurons. It is then processed by a pain matrix of multiple brain regions, resulting in both sensory-discriminative and affective pain perception ([3], reviewed in [20]). Pain pathways in the CNS are modulated by endogenous opioid peptides and arachidonic acid metabolites, acting through G-protein coupled receptors (opioid receptors and cannabinoid receptors) to limit neuronal excitability. GABAergic pathways also act to regulate the excitability of neuronal circuits involved in pain perception.