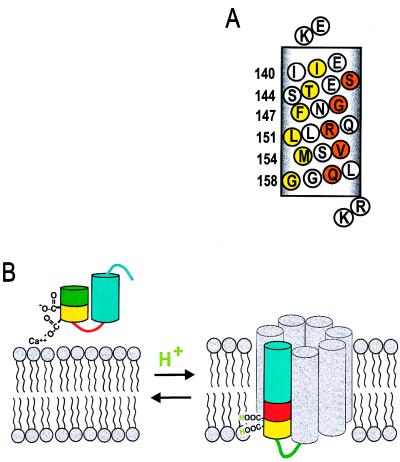
Figure 7.
(A) Helical net representation of annexin XII residues 138–158 at low pH. Residues corresponding to maxima in Π(O2) are in yellow and to maxima in Π(NiEDDA) are in orange. Flanking basic residues (K137 and R159/K160) are proposed to stabilize the transmembrane topography by interacting with the head groups of the acidic phospholipids. (B) Model for the pH-triggered membrane insertion of helices D–E in annexin XII monomer. At neutral pH in solution, or adsorbed to the membrane in the presence of Ca2+, helices D (green/yellow) and E (blue) form a helical hairpin with a short connecting loop (red). In the surface-bound state, Ca2+ is jointly coordinated by Glu-142 and phosphatidylserine (Left). When the pH is lowered, the carboxylate groups are protonated, making it possible for them to be inserted into the bilayer interior as a transmembrane helix (Right). The gray helices are speculative (see text) and are included to represent the remaining portion of the annexin monomer. The number of gray helices in the model is arbitrary.