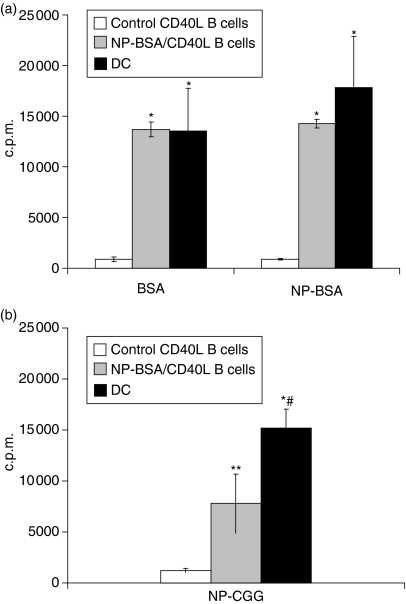
Figure 5.
Antigen-specific CD40L-activated B cells efficiently endocytose and present protein antigen to primed primary T cells. Mice were injected with PBS/CFA or NP-BSA/CFA as in Fig. 4. Ten days after the last treatment, mice were killed and splenocytes cultured for a minimum of 10 days on CD40L-transfected L cells in the presence of rIL-4. CD40L-activated B cells from PBS/CFA-treated (control) mice, CD40L-activated B cells from NP-BSA/CFA-immunized mice, or DC from control mice were pulsed with 1 μg/ml BSA or NP-BSA (a), or NP-CGG (b), irradiated, and 5 × 104 cells added to 105 purified T cells from NP-BSA-immunized mice. After 36 hr, 3H-thymidine was added and c.p.m. incorporation determined 18–20 hr thereafter. Data are presented as means from three independent cultures of each APC (3H-thymidine incorporation in the presence of peptide – 3H-thymidine incorporation in the absence of peptide) ± SE. Background incorporation of T cells cultured in the presence of APC but without peptide averaged <600 c.p.m. and <2800 c.p.m., respectively. Significant differences relative to responses generated in the presence of antigen-pulsed, control CD40L-activated B cells, *P < 0·003 and **P < 0·02, respectively. Significant difference relative to responses generated in the presence NP-CGG-pulsed CD40L-activated B cells from NP-BSA/CFA-immunized mice, #P < 0·03.