Abstract
Background
Most studies of the impact of alcohol dependence on the brain have examined individuals in treatment. Such samples represent a small proportion of alcoholics in the general population. Such samples may embody a bias (‘Berkson’s fallacy’) if the association between variables (for example, alcoholism and cortical gray matter loss) differs between the population of alcoholics in treatment and alcoholics in the general population. Our objective was to determine if treatment-naive alcoholics show structural brain changes versus controls, and to compare our findings with reports evaluating alcoholic samples drawn from treatment populations.
Methods
Structural MRI was used to assess whole brain and regional volumes of cortical gray matter and white matter in 24 young to middle-aged treatment-naive alcohol dependent males versus 17 controls.
Results
Cortical gray matter volumes in alcohol dependent individuals were negatively associated with age and lifetime duration of alcohol use (which were highly confounded). These subjects showed reduced whole brain (p < .05), prefrontal (p < .01), and parietal (p < .05) cortical gray matter compared to controls. White matter and temporal cortex, tissues that usually show volume reductions in samples drawn from treatment, did not differ between treatment-naive alcoholics and controls (all p’s > .40).
Conclusions
Our findings are consistent with the hypothesis that structural brain changes in treatment-naive alcoholics are less severe than those reported in clinical samples of alcoholics, perhaps due to less concomitant psychopathology and a reduced severity of alcoholism in treatment-naive alcoholics. However, caution must be taken when comparing our findings with results from clinical samples, as we did not directly compare treatment-naive alcoholics with treated alcoholics, and our treatment-naive sample tended to be younger than the (clinical) samples reported in the literature. Nevertheless, we suggest that most of the reports of the CNS consequences of alcoholism may not accurately describe the majority of alcoholic dependent individuals.
Keywords: MRI, alcoholic, atrophy, cortical, frontal lobe
INTRODUCTION
The first clues to the association between diseases and both their antecedents and their consequences often derive from the study of select samples of hospitalized patients and autopsy cases. This is the case regarding our knowledge of the effects of alcoholism on brain structure and function. Since not all alcoholics are equally likely to be in these study samples, bias may result when the associations found in these samples are presumed to apply to the population at large. This type of bias, known as Berkson’s fallacy {Berkson, 1946 #3160;Berkson, 1955 #3161}, occurs whenever the association between variables (for example, alcoholism and impaired brain function) differs between the population from which the sample derives (hospitalized alcoholics or alcoholics in treatment) and the general population.
Fleiss {Fleiss, 1973 #1681} presents examples of this bias, and the mathematics underlying it. A classic example of Berkson’s fallacy occurred in Pearl’s {Pearl, 1929 #3165} finding of a negative association between the presence of cancer and tuberculosis in autopsy cases. Pearl found that tuberculosis was less frequent in autopsy cases with cancer than in cases without cancer. He inferred that the same negative association should apply to live patients, and proposed to treat terminal cancer patients with tuberculin in an attempt to arrest their cancer. Pearl ignored the fact that it is improper to extrapolate an association found in autopsy cases to live patients unless all deaths are equally likely to be autopsied.
Roberts et al. {Roberts, 1978 #3166} published the first study empirically demonstrating Berkson’s fallacy as it affects the association between respiratory disease and locomotor disease. In Robert’s sample, 257 individuals (of a random community sample of 2,784 individuals) were hospitalized during the prior six months. There was a very large positive association between the presence of respiratory disease and the presence of locomotor disease in the hospitalized individuals. However, Roberts found that respiratory and locomotor diseases were essentially independent in the entire random sample. The spurious association between respiratory disease and locomotor disease arose in the hospitalized group because the admission rate of people with both diseases (29%) was about three times the rate of people with only respiratory or locomotor disease or neither disease (7–10%). As Fleiss {Fleiss, 1973 #1681} succinctly stated: “… Unless something is known about differential hospitalization rates…, a good amount of skepticism should be applied to any generalization from associations found for hospitalized patients … to associations for people at large.”
Parnas and Teasdale {Parnas, 1987 #3162} presented an example of Berkson’s fallacy in the study of schizophrenia with direct applicability to alcoholism research. An American-Danish prospective study of children of schizophrenic mothers compared psychiatrically hospitalized and untreated cases of schizophrenia spectrum disorders on a number of characteristics. Hospitalized and untreated cases were similar on a number of measures; however, hospitalized individuals exhibited higher levels of substance abuse, affective symptoms, and psychopathic tendencies. The authors suggest that “the clinical population may not be representative of the diagnostic category in question owing to [a greater] co-existence of confounding symptomatology (Berkson’s fallacy)”.
Drawing convenience samples from treatment populations could create an analogous situation in the study of alcohol dependence. Coexisting pathology (e.g., depression or bipolar affective disorder, antisocial personality disorder, attention deficit hyperactivity disorder, posttraumatic stress disorder, and other substance abuse disorders) may be greater in the treatment population than in alcoholics in the general population. This coexisting pathology may not be severe enough to result in clinical diagnoses that would exclude subjects from “alcoholism” research samples. Alternatively, the bias due to Berkson’s fallacy may result if the severity of alcoholism is greater in clinical versus general population samples. Again, in either case, associations found in clinical samples may not generalize to alcoholics in the general population.
How big is this potential bias? Its magnitude depends on the proportion of alcoholics who are in the treatment population. The most current data available indicate that the number of alcoholics in treatment is a small proportion of alcoholics in the general population. The 1992 National Longitudinal Alcohol Epidemiologic Survey {Grant, 1994 #3182} estimates that over 27 million Americans exhibit alcohol abuse or alcohol dependence, or both. At about the same time, Harwood et al., {Harwood, 1994 #3183} estimated that there were approximately 1.8 million Americans receiving treatment for alcohol problems in non-Federal hospital and community based treatment settings. Grant {Grant, 1994 #3169} estimates that only one in 10 individuals who need treatment for alcohol abuse problems has sought treatment. These estimates derive from different methodologies and sampling plans; however, even assuming that three times the 1.8 million individuals from the Harwood study received some form of treatment for alcoholism, the treatment population is still less than a quarter of the population with alcohol problems. Therefore, estimates drawn from clinical samples may not represent up to three-quarters of the individuals with alcohol problems.
Reports of structural imaging abnormalities associated with chronic alcohol abuse and dependence abound in the recent literature, but most (if not all) of the samples have been drawn from alcohol treatment centers {Pfefferbaum, 1995 #2911;Shear, 1994 #1722;Harper, 1988 #1723;de la Monte, 1988 #3178;Jernigan, 1992 #3180;Pfefferbaum, 1998 #3179;Sullivan, 1995 #772;Jernigan, 1991 #2898;Pfefferbaum, 1992 #827}. This study focuses on structural brain imaging in young to middle-aged treatment-naive alcohol dependent males versus controls. We compare our results to the findings presented in the literature for alcohol dependent samples drawn from treatment populations.
METHODS
Subjects
Subjects were recruited from the community (via advertisements for “light drinkers” and “heavy drinkers”) for an alcohol administration Magnetic Resonance Spectroscopy (MRS) study {Fein, 2000 #3164;Estilaei, 2001 #3168;Goldmann, 2000 #3170} investigating 1H MRS visibility of alcohol, and the effect of alcohol tolerance on MRS alcohol visibility. Forty-one individuals, 24 heavy drinkers (HD) and 17 light drinkers (LD), were studied according to protocols approved by the local Institutional Review Board. Table 1 shows the demographic, family history, and alcohol use information for these subjects. All HD subjects satisfied DSM-IV-R criteria for lifetime dependence on alcohol. Subjects were screened to exclude individuals with a current or past history of substance abuse (other than alcohol) or major psychiatric or neurologic disorder (including history of head injury with loss of consciousness). Subjects were also screened to exclude individuals with a current or past history of medical conditions that may affect brain structure, (including diabetes, HIV, and lung, kidney, or heart disease). Lifetime drinking history was ascertained using the time-line followback method {Sobell, 1992 #1310}. Family history of alcoholism was assessed using a family tree questionnaire {Sobell, 1985 #2674}. Subjects with a father or mother identified as a “problem drinker” were considered family history-positive; subjects with only siblings or second-degree relatives identified as “problem drinkers” were not considered family history-positive. A hand-held Breathalyzer was used to confirm sobriety at the time of the magnetic resonance study (MRI).
Table 1.
Demographic and Alcohol Use Variables
| Light Drinkers (n=17) | Heavy Drinkers (n=24) | |||
|---|---|---|---|---|
| Mean±SD | Range | Mean ±SD | Range | |
| Age (yrs) | 30.0±5.2 | 23–44 | 38.7±9.0 | 25–54 |
| Education (yrs) | 16.4±2.2 | 14–20 | 14.5±1.3 | 12–17 |
| Ethnicity a | 16 C, 1AA | 20C, 2AA, 1 NA, 1A | ||
| Positive Family History of Alcoholism | 19%(n=16) | 59%(n=22) | ||
| Lifetime duration of drinking (mos) | 171.8±63.2 | 72–324 | 284.5±114.9 | 122–564 |
| Average consumption (drinks b per month) | 25.9±14.3 | 1–65 | 203.4±119.6 | 48–540 |
| Lifetime total number of drinks | 4,219±2,385 | 216–10,205 | 58,583±48,041 | 14,508–181,440 |
| Peak consumption (drinks per month) | 57.9±39.7 | 1–160 | 415.1±285.2 | 90–1,020 |
| Duration of peak consumption (mos) | 51.3±48.0 | 5–216 | 104.5±125.3 | 6–528 |
| Average consumption in the last 6 months (drinks per month) | 30.3±27.9 | 1–96 | 341.0±274.9 | 90–1,020 |
C = Caucasian, AA = African American, NA = Native American, and A = Asian
One drink was considered an alcoholic beverage containing approximately 11 g of alcohol.
MR Measurements
All image acquisition and analysis procedures have been described in detail previously {Fein, 2000 #3126}.
Image Acquisition
All measurements were carried out on a whole body 1.5 T Magnetom Vision system equipped with a standard quadrature head coil. A vacuum-molded head holder was used to minimize motion of the subject’s head. The structural imaging protocol (before alcohol administration) consisted of: (a) a sagittal T1-weighted localizer sequence, (b) an oblique axial double spin-echo sequence (TR/TE1/TE2 = 3000/20/80 ms), angulated at −10 degrees from the planum sphenoidale. This study yielded both a T2-weighted and a proton-density weighted (PD) image, and covered the entire brain in contiguous 3 mm thick slices. These slices were obtained in an interleaved manner, with an in-plane resolution of .94 × .94 mm. (c) an oblique coronal T1-weighted gradient echo sequence (3D MP-RAGE, TR/TE = 9.7/4.6 ms), angulated perpendicular to the optic nerve, with a 1.5 mm slice thickness, and an in-plane resolution of 1 × 1 mm.
Image Processing
The tissue volumes in the segmented image were obtained using computer-assisted methods. The image-processing technician was blind to subject demographics and group membership. The T1, PD, and T2-weighted images were used for segmentation into tissue categories. The segmentation process began with automated stripping of the skull from the images, inhomogeneity filtering of the spin-echo images, and coregistration of the T1-weighted images to the spin-echo data set. Next, the operator chose very conservative samples of cerebrospinal fluid (CSF), white matter, and gray matter as seeds for a K-Means cluster analysis {SAS, 1988 #2538} that assigned all of the pixels in the brain to these three tissue categories. This was followed by manual editing of the axial segmented images on a slice-by-slice basis to separate cortical from subcortical gray matter and ventricular from sulcal CSF. The technician also reclassified pixels as white matter signal hyperintensity (WMSH) that had been classified by the K-means procedure as either gray matter or CSF (due to their relative hyperintensity), but were clearly white matter by anatomic location. We have recently demonstrated the reliability and validity of this approach to image segmentation {Cardenas, 2001 #3125}
Regional Processing in the Talairach Coordinate System
In the Talairach coordinate system, the brain is subdivided into a 12 (superior-inferior) X 9 (anterior-posterior) X 8 (lateral) grid, with a total of 864 voxels. The Brodmann areas encompassed by each of these 864 voxels are identified in the 1988 Talairach atlas {Talairach, 1988 #1252}. We defined 15 cortical regions of interest (ROI) by their corresponding Brodmann areas: orbital frontal and frontal pole (10, 11), posterior prefrontal (6, 8, 44), dorsolateral prefrontal (9, 46), lateral prefrontal (45, 47), primary motor (4), primary sensory (1, 2, 3), lateral parietal (39, 40), mesial parietal (5, 7, 23, 31), anterior occipital (37), visual association (17, 18, 19), anterior temporal (21, 38), superior temporal (34, 41, 42), inferior temporal (20), anterior cingulate (24, 32), and the limbic lobe (24, 29). The ensuing transformation of each subject’s T1-weighted image to the Talairach coordinate system involved piecewise linear transformations of 12 compartments for each subject’s brain. The resulting ROIs were specific to each subject, but reflect a common Talairach definition. The final regional tissue volumes resulted from superimposing the subject-specific ROI on the subject’s segmented image (and counting the segmented pixels). This method has been described in detail elsewhere {Fein, 2000 #3126;Cardenas, 2001 #3125}.
Statistics
Statistical analyses were performed using the ‘proc GLM’ routine in the SAS™ software package {SAS, 1988 #2538}. Comparisons of structural imaging variables between HD and LD subjects were performed with intracranial vault volume (ICV) as a covariate. The group difference effect size (percent of variance of the imaging variable accounted for by group membership) was computed after removing imaging variable variance due to inter-subject differences in ICV. Associations within each group of demographic and drinking variables with imaging measures were analyzed using partial correlations (with ICV always partialled out, and other variables partialled out as indicated). LD was compared to the entire HD sample, and to a subset of HD of comparable age (i.e., HD subjects over 42 years of age excluded).
RESULTS
Demographic and Alcohol Use Variables
The LD sample was younger than the HD sample (t37.7 = 3.90, p = .0004), and had about two years more education (t23.6 = 3.20, p = .004). The LD had a lower proportion of individuals who were family history positive for alcoholism (19% versus 59%, chi-square = 6.18, p = .013). Since the LD and HD samples were selected to be very different on alcohol use variables, statistical comparisons on those variables are not appropriate. (Lifetime alcohol consumption for HD subjects was about 14 times that of LD subjects.)
We present the following analyses both for the entire HD sample (which was, on average, about nine years older than the LD sample), and for an HD subgroup comparable in age to the LD sample. The comparison of LD to the entire HD sample provides the best estimates of the associations between age and the structural imaging variables. The comparison of the LD to an age-comparable HD subgroup examines the brain structure of untreated heavy drinkers versus light drinkers, without the confounding effect of age differences between the samples. Table 2 presents whole brain and regional cortical gray matter volume differences for LD versus both the entire HD sample, and the age-comparable HD subgroup.
Table 2.
Whole Brain and Regional Gray Matter
| Light Drinker sample vs. Heavy Drinker sample |
Light Drinker sample vs. Age-comparable Heavy Drinker subgroup |
|||
|---|---|---|---|---|
| % difference from Light Drinkers | % of variance explained by group membershipa | % difference from Light Drinkers | % of variance explained by group membership a | |
| Total Cortical Gray Matter | −4.6 | 14.7* | −1.4 | 3.2 |
| Prefrontal Lobe | ||||
| Orbital Frontal and Frontal Pole | −0.1 | 0.2 | 1.4 | 1.0 |
| Lateral Prefrontal | −3.0 | 3.9 | −0.4 | 3.9 |
| Posterior Prefrontal | −8.3 | 19.6** | −6.6 | 14.8* |
| Dorsolateral Prefrontal | −8.8 | 17.2** | −7.1 | 13.8* |
| Parietal Lobe | ||||
| Lateral Parietal | −7.8 | 11.4* | −5.0 | 6.4 |
| Mesial Parietal | −7.1 | 15.6* | −4.9 | 9.0 |
| Motor Cortex | −4.8 | 6.3 | −3.0 | 2.5 |
| Sensory Cortex | −7.6 | 9.0 | −5.3 | 4.7 |
| Occipital Lobe | ||||
| Anterior Occipital | −4.5 | 7.0 | −1.8 | 2.2 |
| Visual Cortex and Association Cortex | −4.6 | 4.6 | −3.7 | 4.4 |
| Temporal Lobe | ||||
| Anterior Temporal | −3.2 | 5.4 | −1.4 | 1.2 |
| Superior Temporal | −4.4 | 6.9 | −1.0 | 0.6 |
| Inferior Temporal | 1.3 | 0.2 | 3.7 | 2.5 |
| Anterior Cingulate | −1.0 | 0.1 | 3.0 | 1.6 |
| Limbic Lobe | −0.3 | 0.0 | 4.6 | 2.0– |
after removal of variance associated with ICV volume.
p < 0.05
p < 0.01.
Structural Imaging Measures
LD sample vs. HD sample
The entire HD sample did not differ in ICV volume from LD (p > .15). HD showed reduced total cortical gray matter (45.6% versus 46.8%, p < .02) and increased WMSH volume (Wilcoxon z approximation = 1.65, one-tail p < .05) compared to LD. The posterior prefrontal and dorsolateral prefrontal cortex were reduced in HD vs. LD (p’s < .01), as was the lateral and mesial parietal cortex (p’s < .05). Sulcal CSF was increased in all regions of reduced gray matter. This is in contrast to both total and regional white matter measures which remained virtually identical between LD and HD (all p’s > .52). Finally, we divided the gray matter regions into right and left hemisphere volumes, and re-analyzed the data using hemisphere as a within-subject factor. There was no evidence for a Side X Group or a Side X Region X Group effect (both F’s < 1.20, p’s > .32).
LD sample vs. age-comparable HD subgroup
We selected an HD subgroup (n=16) that was comparable in age to the LD sample (by eliminating all HD subjects over age 42). There were no differences between the age-comparable HD subgroup and LD for any of the whole brain volume measures (cortical gray matter, white matter, sulcal CSF, ventricular CSF, or WMSHs, all p’s > .32). The only structural imaging differences between LD and the age-comparable HD subgroup were reduced cortical gray matter in the posterior prefrontal cortex and dorsolateral prefrontal cortex (p’s < .05).
Association of Imaging Variables with Age and Alcohol Use
Within LD, there was a trend toward an association of age with total cortical gray matter (r =−.48, p = .07) and total sulcal CSF (r = .49, p = .07). There were no associations of age or lifetime duration of alcohol use with any regional structural imaging variables (e.g., posterior prefrontal and dorsolateral prefrontal gray matter; r’s < |.14|, p’s > .60).
In the HD sample, there was a strong negative correlation of both age and lifetime duration of alcohol use with total cortical gray matter volume (both r’s < − .79, p’s < .0001). Correlations between age and regional cortical gray matter volume and duration of alcohol use and regional cortical gray matter volume were nearly identical, ranging from − .47 to − .75 for 12 of the 15 cortical regions (excepting the anterior and inferior temporal cortex and visual cortex). The correlations with age and lifetime duration of alcohol use for sulcal CSF measures (total and regional) were similar to those observed for cortical gray matter measures, although of opposite sign.
In the age-comparable HD subgroup there were also negative correlations of age and lifetime duration of alcohol use with total cortical gray matter (r = − .73, p = .003, and r = − .81, p = .0004). Correlations between age and regional cortical gray matter ranged from − .54 to − .73 for seven of the 15 regions (posterior prefrontal, dorsolateral prefrontal, lateral prefrontal, mesial parietal, lateral parietal, motor, and sensory cortex), and correlations between lifetime duration of alcohol use and regional cortical gray matter ranged from − .56 to − .88 for the same seven of the 15 cortical regions, with the addition of visual cortex.
Age was confounded with lifetime duration of drinking in LD (r = .59, p = .02), and almost completely confounded with lifetime duration of alcohol use in both the entire HD sample (r = .91, p < .0001), and in the age-comparable HD subgroup (r = .86, p < .0001). When we attempted to disentangle the association of the imaging variables with age vs. with the association of duration of alcohol use by partialling out the variance associated with one of the variables (age or duration of drinking), and then examining the remaining association of the other variable, we found no associations between age or drinking duration with any imaging measure. Figure 1 presents the posterior prefrontal gray matter data (i.e., the variable with the largest group difference effect) for all subjects in LD and HD, illustrating both the volume reduction in HD versus LD, and the association in HD of this brain volume with subject age and with lifetime duration of alcohol use.
Figure 1.
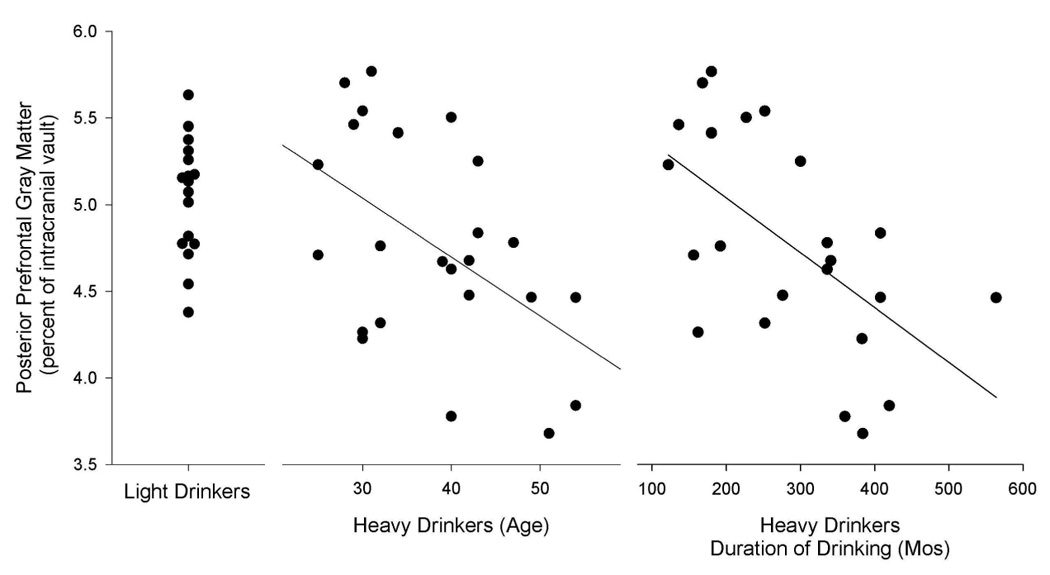
posterior prefrontal gray matter (as a percent of the intracranial vault) for LD and HD. This is the regional variable with the largest difference between LD and HD. This figure illustrates both the volume reduction in HD versus LD, and the association in HD of this regional brain volume with both subject age and lifetime duration of alcohol use. We note that the data for HD is presented twice, once in relation to age and again in relation to duration of alcohol use.
There were no associations of average or peak alcohol dose with any of the structural imaging variables, except for WMSH volume (r = .43, p < .05). Finally, the entire HD sample showed no association of any imaging variable with family history of alcoholism, or with years of education (all r’s < |.20|, p’s > .30).
DISCUSSION
We found cortical gray matter volume reductions (primarily in prefrontal and parietal regions) in male heavy drinkers who met criteria for alcohol dependence, but had never been in treatment. The cortical volume loss was not correlated with family history of alcoholism or with education, suggesting that the volume loss reflects the effect of alcohol ingestion, rather than premorbid factors that predispose subjects to heavy drinking. Unlike most other studies of structural brain changes (which used samples drawn from treatment), this treatment-naive sample showed no white matter {Pfefferbaum, 1995 #2911;Shear, 1994 #1722;Harper, 1988 #1723;de la Monte, 1988 #3178;Jernigan, 1992 #3180} or temporal lobe {Jernigan, 1991 #2898;Pfefferbaum, 1992 #827;Pfefferbaum, 1997 #2989;Sullivan, 1995 #772} volume loss. Our findings are consistent with the hypothesis that structural brain changes in treatment-naive alcoholics are less severe than those reported in clinical samples of alcoholics. However, caution must be taken when comparing our findings with results from clinical samples, as we did not directly compare treatment-naive alcoholics with treated alcoholics. In addition, our treatment-naive sample tended to be younger than the (clinical) samples reported in the literature. Given that increasing age magnifies the effects of alcohol on brain structure and function {Carlen, 1978 #1068;Wilkinson, 1985 #918;Jernigan, 1986 #884;Lishman, 1987 #1083;Ron, 1983 #1087}, the younger age of our sample compared to clinical samples in the literature may contribute to the less morbid structural imaging findings in our study.
Preferential prefrontal gray matter atrophy associated with alcohol abuse was first suggested by Courville {Courville, 1955 #871} in 1955. Prefrontal atrophy has since become one of the most frequent findings in investigations of the CNS effects of alcohol dependence {Harper, 1990 #812;Pfefferbaum, 1997 #2989;Gilman, 1990 #1794;Adams, 1993 #1803;Pfefferbaum, 1995 #2911}. Preferential frontal lobe involvement in alcoholism has also been documented with PET {Wang, 1993 #1715;Volkow, 1992 #1716;Risberb, 1987 #3172}, and in studies of cortical neuronal counts {Kril, 1994 #3173;Harper, 1989 #811;Harper, 1987 #810;Harper, 1990 #812;Kril, 1989 #887}.
While our strongest findings were in the prefrontal cortex, parietal cortex was also adversely affected by heavy drinking. Jernigan also found parietal cortex atrophied in a MRI study of abstinent alcoholics {Jernigan, 1991 #2898}. Parietal gray matter volume reductions are consistent with the frequent findings of alcohol-related impairments in visuo-spatial abilities and sensory integration {Sullivan, 2000 #3191}.
We did not observe lateralized reductions in cortical volume. This does not support the right hemisphere model of the effects of alcoholism on brain function {Hutner, 1996 #3181}. Purported right hemisphere tasks are often novel tasks requiring new learning, which is dependent on prefrontal cortex. They also involve spatial (rather than verbal) processing of stimuli, which is dependent on parietal cortex. We hypothesize that ‘right hemisphere impairment’ is actually impairment in functions subserved by prefrontal and parietal cortices.
There was no evidence of white matter loss in the HD sample. This is in contrast to studies of alcohol dependent samples drawn from treatment settings where white matter loss is a common finding. (However, as noted above, this study is not a direct comparison of treatment-naïve and clinical samples of alcoholics.) Our finding of intact white matter volume on structural MRI in these treatment-naive subjects occurred in the context of a 13% reduction in the 31P MRS broad component measured in the white matter of a subset of the HD subjects compared to LD {Estilaei, 2001 #3168}. This suggests higher rigidity of white matter phospholipids in the absence of white matter volume loss. Our hypothesis is that alterations in the composition of membrane lipids lead to changes in myelin structure, and, eventually, to tissue volume loss. If this is true, then 31P MRS broad component measures should be even more reduced in clinical samples, where they occur in the presence of white matter volume reductions. We also found 1H MRS evidence for pre-atrophic white matter n-acetylaspartate (NAA) reductions in a subset of the HD sample compared to LD {Goldmann, 2000 #3170}. Similar to the argument regarding 31P MRS measures, we hypothesize that the white matter NAA reductions will be larger in clinical samples where they are likely to occur in the context of white matter volume loss.
In the HD sample (and the age-comparable HD subgroup) we found strong correlations of reductions in total and regional cortical gray matter volume with age and with lifetime duration of alcohol use; however, age and duration of alcohol use were almost totally confounded. LD showed a strong trend toward association of age with total cortical gray matter, but not with any regional gray matter measures. This suggests that long-term alcohol dependence as the most likely cause of the prefrontal gray matter volume reductions in the HD sample. However, we cannot definitively say whether the volume reductions in HD are associated with long-term heavy alcohol use together with increasing age, or simply with duration of alcohol use. Given that the older drinkers have a longer duration of alcohol use, the simplest interpretation of the data would be that duration of alcohol use is the operative factor in cortical volume loss in alcohol dependent individuals. This interpretation is not consistent with the literature, which tells us that there is a greater degree and persistence of irreversible brain atrophy in older compared to younger alcoholics, independent of the duration of their drinking {Fein, 1990 #767}.
There may be other manifestations of the bias that occurs when findings of studies of treated alcoholics are presumed to apply to all alcoholics. It is possible that there is greater psychiatric comorbidity in clinical samples than in treatment-naive samples of alcoholics. We know that comorbidity of substance use and psychiatric disorders is substantial. The Epidemiology Catchment Area (ECA) Study {Narrow, 1993 #3188} found a history of psychiatric disorder in 35% of the 13.5% of the general population who had a history of alcohol abuse. The ECA estimates on comorbidity of psychiatric and alcohol abuse disorders has been replicated by the more recent National Comorbidity Survey data {Kessler, 1994 #3186}. Among individuals in alcohol and drug abuse treatment, estimates are that up to 80% have psychiatric symptoms {Kosten, 1988 #3187}. These epidemiological data address coexisting psychopathology that is severe enough to meet criteria for a psychiatric disorder. This is an underestimate of coexisting psychopathology in that it does not address pathology that is of insufficient severity to result in a clinical diagnosis (e.g., depression or bipolar affective symptoms, antisocial personality traits, attention deficit hyperactivity disorder traits, posttraumatic stress disorder symptoms, and other substance abuse history). Although we did not directly assess psychiatric comorbidity in the study sample, we hypothesize that treatment-naïve samples have less psychiatric comorbidity than clinical samples of alcoholics.
We only examined men in this study. The bias inherent in studying clinical populations may be different for men and women, and the greater CNS consequences reported for female versus male clinical samples {Jacobson, 1986 #1079;Bergman, 1987 #764} may reflect this difference. The more morbid CNS findings for women may be spurious if clinical samples of alcoholic women differ from treatment-naive alcoholic women more than do clinical versus treatment-naive samples of alcoholic men. This is entirely possible, since women (for a variety of reasons) are less likely than men to receive treatment for alcohol problems [Conference on Substance Abuse and the American Woman at the Center on Addiction and Substance Abuse at Columbia University, 1996]. Therefore, clinical samples versus treatment-naive samples of female alcoholics may differ in severity of alcoholism or prevalence and severity of concomitant psychopathology than do clinical versus treatment-naive samples of male alcoholics.
Alcoholics in treatment need to be compared directly to non-treatment seeking alcohol dependent individuals to determine whether clinical samples differ from treatment-naive samples on measures of CNS structure and function. Coexisting psychopathology, prevalence and severity of predisposing factors (which may be associated with premorbid abnormalities in CNS structure and function), and severity of alcoholism should be assessed. Finally, we need to examine the “more vulnerable” female alcoholic pictured in the literature; are these findings a function of a bias toward sicker individuals in female versus male clinical populations?
Acknowledgments
This work was supported by Grants AA10788 (DJM) and AA11311 (GF), both from the National Institute of Alcoholism and Alcohol Abuse.
REFERENCES
- Bergman H. Brain dysfunction related to alcoholism: Some results from the KARTAD project. In: Parsons OA, Butters N, Nathan P, editors. Neuropsychology of Alcoholism: Implications for Diagnosis and Treatment. New York: Guilford Press; 1987. pp. 21–45. [Google Scholar]
- Berkson J. Limitations of the application of fourfold table analysis to hospital data. Biom. Bull. (now Biometrics) 1946;2:47–53. [PubMed] [Google Scholar]
- Berkson J. The statistical study of association between smoking and lung cancer. Proc. Staff Meet. Mayo Clin. 1955;30:319–348. [PubMed] [Google Scholar]
- Caetano R, Clark C, Greenfield T. Prevalence, trends, and incidence of alcohol withdrawal symptoms: Analysis of general population and clinical samples. NIAAA's Epidemiologic Bulletin No. 38. 1998;22(1):73–79. [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Ezekiel F, Di Sclafani V, Gomberg B, Fein G. Reliability of tissue volumes and their spatial distribution for segmented magnetic resonance images. Psychiatry Research: Neuroimaging. 2001;106:193–205. doi: 10.1016/s0925-4927(01)00075-0. [DOI] [PubMed] [Google Scholar]
- Carlen PL, Wortzman G, Holgate RC, Wilkinson DA, Rankin JG. Reversible cerebral atrophy in recently abstinent chronic alcoholics measured by computed tomography scans. Science. 1978;200:1076–1078. doi: 10.1126/science.653357. [DOI] [PubMed] [Google Scholar]
- Courville CB. Effects of Alcohol on the Nervous System of Man. Los Angeles: San Lucas Press; 1955. [Google Scholar]
- de la Monte SM. Disproportionate atrophy of cerebral white matter in chronic alcoholics. Archives of Neurology. 1988;45:1990–1992. doi: 10.1001/archneur.1988.00520330076013. [DOI] [PubMed] [Google Scholar]
- Estilaei MR, Matson GS, Payne GS, Leach MO, Fein G, Meyerhoff DJ. Effects of chronic alcohol consumption on the broad phospholipid signal in human brain: an in vivo 31P MRS study. Alcoholism: Clinical and Experimental Research. 2001;25:89–97. [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Tanabe J, Cardenas V, Weiner MW, Jagust WJ, Reed BR, Norman D, Schuff N, Kusdra L, Greenfield T, Chui H. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55:1626–1635. doi: 10.1212/wnl.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Meyerhoff DJ. Ethanol in human brain by magnetic resonance spectroscopy: correlation with blood and breath levels, relaxation and magnetization transfer. Alcoholism: Clinical and Experimental Research. 2000;24:1227–1235. [PMC free article] [PubMed] [Google Scholar]
- Fleiss J. Statistical Methods for Rates and Proportions. New York: Wiley; 1973. [Google Scholar]
- Goldmann H, Tolou-Shams M, Salas G, Fein G, Meyerhoff DJ. 1H MRS in the brain of light and heavy drinkers: Alcohol and neurons; International Society of Magnetic Resonance in Medicine Seventh Annual Meeting; 2000. [Google Scholar]
- Grant B. Epidemiologic Bulletin No. 35: Prevalence of DSM-IV alcohol abuse and dependence, United States 1992. Alcohol Health & Research World. 1994;18:243–248. [PMC free article] [PubMed] [Google Scholar]
- Grant B. The influence of comorbid major depression and substance use disorders on alcohol and drug treatment: results of a national survey. Rockville, MD: National Institute on Drug Abuse Technical Review Meeting: Comorbid Mental and Addictive Disorders: Treatment and HIV-Related Issues. 1994
- Harper C, Kril J. Patterns of neuronal loss in the cerebral cortex in chronic alcoholic patients. Journal of the Neurological Sciences. 1989;92:81–89. doi: 10.1016/0022-510x(89)90177-9. [DOI] [PubMed] [Google Scholar]
- Harper C, Kril J, Daly J. Are we drinking our neurones away? British Medical Journal. 1987;294:534–536. doi: 10.1136/bmj.294.6571.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Kril JJ. Neuropathology of Alcoholism. Alcohol & Alcoholism. 1990;25:207–216. doi: 10.1093/oxfordjournals.alcalc.a044994. [DOI] [PubMed] [Google Scholar]
- Harper CG, Kril JJ, Daly JM. Brain shrinkage in alcoholics is not caused by a change in hydration - a pathological study. Journal of Neurology Neurosurgery and Psychiatry. 1988;51:613–615. doi: 10.1136/jnnp.51.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood H, Thomson M, Nesmith T. Healthcare Reform and Substance Abuse Treatment: The Cost of Financing Under Alternative Approaches. Fairfax, VA: Lewin-VHI; 1994. [Google Scholar]
- Hutner N, Oscar-Berman M. Visual laterality patterns for the perception of emotional words in alcoholic and aging individuals. J Stud Alcohol. 1996;57:144–154. doi: 10.15288/jsa.1996.57.144. [DOI] [PubMed] [Google Scholar]
- Jacobson R. The contributions of sex and drinking history to CT scan changes in alcoholics. Psychological Medicine. 1986;16:547–559. doi: 10.1017/s003329170001031x. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Butters N, Cermak LS. Studies of brain structure in chronic alcoholism using magnetic resonance imaging. In: Zakhari S, Witt E, editors. Imaging in Alcohol Research. Rockville, MD: NIAAA; 1992. pp. 121–133. [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, Grant I, Schuckit M, Cermak LS. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcoholism: Clinical and Experimental Research. 1991;15:418–427. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Pfefferbaum A, Zatz LM. Computed tomography correlates in alcoholism. In: Grant I, editor. Neuropsychiatric Correlates in Alcoholism. Washington, DC: American Psychiatric Association; 1986. pp. 21–36. [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen H, Kendler KS. Lifetime and 12-Month Prevalence of DSM-III-R Psychiatric Disorders in the United States. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kleber HD. Differential Diagnosis of Psychiatric Comorbidity in Substance Abusers. Journal of Substance Abuse Treatment. 1988;5:201–206. doi: 10.1016/0740-5472(88)90042-6. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Cartwright H, Svoboda M. Neuronal changes in the cerebral cortex of chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1994;18:35A. [Google Scholar]
- Kril JJ, Harper CG. Neuronal counts from four cortical regions in alcoholic brains. Acta Neuropathologica. 1989;79:200–204. doi: 10.1007/BF00294379. [DOI] [PubMed] [Google Scholar]
- Lishman WA, Jacobson RR, Acker C. Brain damage in alcoholism: Current concepts. Acta Medica Scandanavia. 1987;717:5–17. doi: 10.1111/j.0954-6820.1987.tb13037.x. [DOI] [PubMed] [Google Scholar]
- Narrow WE, Regier DA, Rae DS, Manderscheid RW, Locke BZ. Use of services by persons with mental and addictive disorders: Findings from the National Institute of Mental Health Epidemiologic Catchment Area Program. Archives of General Psychiatry. 1993;50:95–107. doi: 10.1001/archpsyc.1993.01820140017002. [DOI] [PubMed] [Google Scholar]
- Parnas J, Teasdale T. A matched-paired comparison of treated versus untreated schizophrenia spectrum cases. A high-risk population study. Acta Psychiatrica Scandinavica. 1987;75:44–50. doi: 10.1111/j.1600-0447.1987.tb02749.x. [DOI] [PubMed] [Google Scholar]
- Pearl R. Cancer and tuberculosis. Am. J. Hyg. (now Am. J. Epidemiol.) 1929;9:97–159. [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcoholism: Clinical and Experimental Research. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a five year interval. Archives of General Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Risberb J, Berglund M. Cerebral blood flow and metabolism in alcoholics. In: Parsons OA, Butters N, Nathan PE, editors. Neuropsychology of Alcoholism. New York: Guilford Publications; 1987. pp. 64–75. [Google Scholar]
- Roberts RS, Spitzer WO, Delmore T, Sackett DL. An empirical demonstration of Berkson's bias. J. Chronic Dis. 1978;31:119–128. doi: 10.1016/0021-9681(78)90097-8. [DOI] [PubMed] [Google Scholar]
- Ron MA. The alcoholic brain: CT scan and psychological findings. Psychological Medicine. 1983 Suppl 3:1–31. doi: 10.1017/s0264180100000345. [DOI] [PubMed] [Google Scholar]
- Saitz R. Introduction to alcohol withdrawal. Alcohol Research & Health. 1998;22(1):5–12. [PMC free article] [PubMed] [Google Scholar]
- SAS II. SAS/SAT User's Guide. Release 6.03 Edition. Cary, NC, USA: SAS Institute; 1988. [Google Scholar]
- Shear PK, Jernigan TL, Butters N. Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 1994;18:172–176. doi: 10.1111/j.1530-0277.1994.tb00899.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-back: A technique for assessing self-reported ethanol consumption. In: Allan J, Litten RZ, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. New Jersey: Mumana Press; 1992. pp. 41–72. [Google Scholar]
- Sobell R, Sobell L, Pavan D. Reliability of family tree questionnaire for assessing family history of alcohol problems. Drug and Alcohol Dependence. 1985;15:61–67. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging, chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism: Clinical and Experimental Research. 2000;24(5):611–621. [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Brain. New York, NY: Theime Medical Publishers; 1988. [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Burr G, Pascani K, Dewey SL, Wolf AP. Decreased brain metabolism in neurologically intact healthy alcoholics. American Journal of Psychiatry. 1992;149:1016–1022. doi: 10.1176/ajp.149.8.1016. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Roque CT, Cestaro VL, Hitzemann RJ, Cantos EL, Levy AV, Dhawan AP. Functional importance of ventricular enlargement and cortical atrophy in healthy subjects and alcoholics as assessed with PET, MRI, and neuropsychologic testing. Radiology. 1993;186:59–65. doi: 10.1148/radiology.186.1.8416587. [DOI] [PubMed] [Google Scholar]
- Wilkinson DA. Neuroradiologic investigations of alcoholism. In: Tarter RE, Van Thiel DH, editors. Alcohol and the Brain: Chronic Effects. New York: Plenum Press; 1985. pp. 183–215. [Google Scholar]


