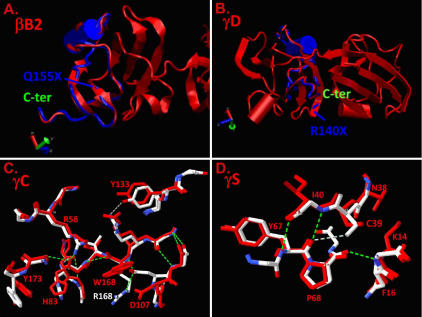
Figure 4.
Molecular modeling of the effects of mutations presented in Table 1 on βγ-crystallin structures. Normal structures are shown in red and the overlaid mutant structures are shown in blue (truncations) and blue and white (substitutions). A: The normal βB2-crystallin structure is shown in red, and the Q155X mutation is predicted to remove the COOH-terminal 51 amino acids shown in the blue overlay. B: The normal γD-crystallin structure is shown in red and the R140X mutation is predicted to remove the COOH-terminal 34 amino acids shown in the blue overlay. C: The normal γC-crystallin structure in the vicinity of the R168R mutation is shown in white with positive charges shown in blue and negative charges in red. The overlaid R168W mutant is shown in red, demonstrating the substitution of the polar arginine residue by the apolar tryptophan and consequent displacement of D107. D: The normal γS-crystallin structure in the vicinity of the fragments of 3D structures of mutant proteins (γC-crystallin) and S39C (γS-crystallin) mutations are shown in red. Hydrogen-bonding patterns of the mutant γC- and γS-crystallin structures are shown by dashed lines (green).