TABLE 1.
One-Pot Diastereoselective Benzopyran Synthesis
| SM | R′M | 2π | Cycloadduct | cis/transa | % | |
|---|---|---|---|---|---|---|
| 1b | 3 | t-BuMgCl 26 |
|
|
NA | 50 |
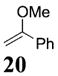
| 2b | 4 | MeLi 27 + MgBr2 |
|
|
~6:1 | 27 |
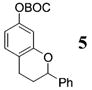
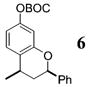
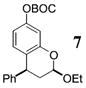
| 3b | 4 | PhMgBr 28 |
|
|
>50:1c | 73 |
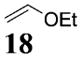
| 4b | 4 |

|
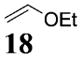
|
|
>50:1c | 70 |
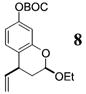
| 5b | 4 |
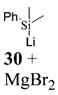
|
|
|
>50:1c | 86 |
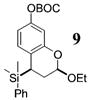
| 6b | 4 | MeMgCl 31 |
|
|
~24:1 | 66 |
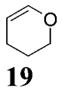
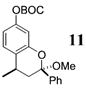
| 7d | 4 | MeMgCl 31 |
|
|
~4:1 | 55 |
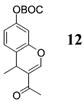
| 8e | 4 | MeLi 27 + MgBr2 |
|
|
NA | 57 |
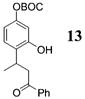
| 9e | 4 | MeLi 27 + MgBr2 |
|
|
NA | 58 |
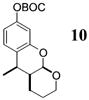
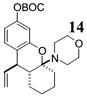
| 10d | 4 |
|
|
|
>50:1c | 70 |
| 11e | 4 | MeMgCl 31 |
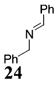
|
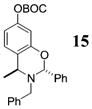
|
<1:50c | 94 |
| 12b | 4 | MeMgCl 31 |
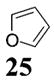
|
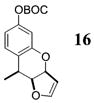
|
>50:1c | 76 |
Cis/trans ratio determined by 1H NMR analysis of crude material.
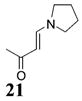
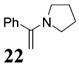
2π component used as the solvent for the reaction.
>50:1 signifies that no other isomers could be found in the 400-MHz 1H NMR spectra.
5–10 equiv of 2π component used.
2–5 equiv of 2π component used.
