FIGURE 2.
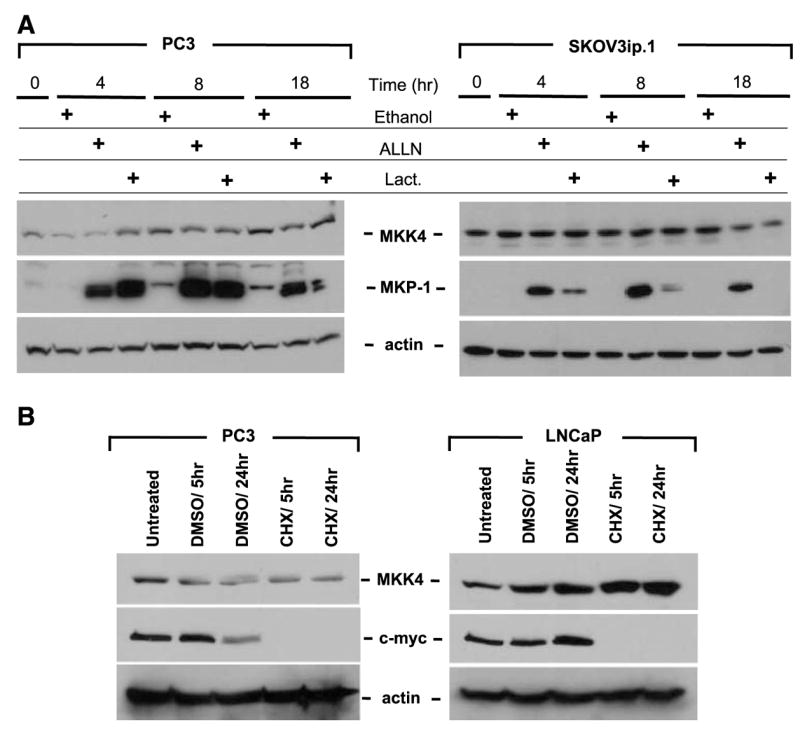
The MKK4 protein is highly stable and inhibition of the proteasome does not increase MKK4 protein levels. A. Cells were incubated with medium supplemented with proteasome inhibitors (ALLN or lactacystin) or vehicle control (ethanol) for the indicated times. Protein lysates were prepared from PC3 and SKOV3ip.1 cells. Total protein (100 μg) was immunoblotted for MKK4. Membranes were stripped and reprobed for MKP-1 as a control for inhibition of proteasome function and subsequently stripped and reprobed for β-actin as a control for protein loading. Loss of MKP-1 expression in the lactacystin 18-h time points may be due to hydrolysis of the covalent linkage between lactacystin and the proteasome (42). Representative data from at least three experiments are shown. B. Treatment of prostate cancer cells with cycloheximide (CHX) does not result in degradation of MKK4 protein. PC3 and LNCaP cells were incubated with cycloheximide or DMSO vehicle control for 5 or 24 h. Top, protein (100 μg) was immunoblotted for MKK4; middle, membranes were stripped and reprobed with an antibody against c-myc as a control for inhibition of protein synthesis; bottom, membranes were stripped and reprobed for β-actin as a loading control. Representative data from at least three experiments are shown.
