Figure 4.
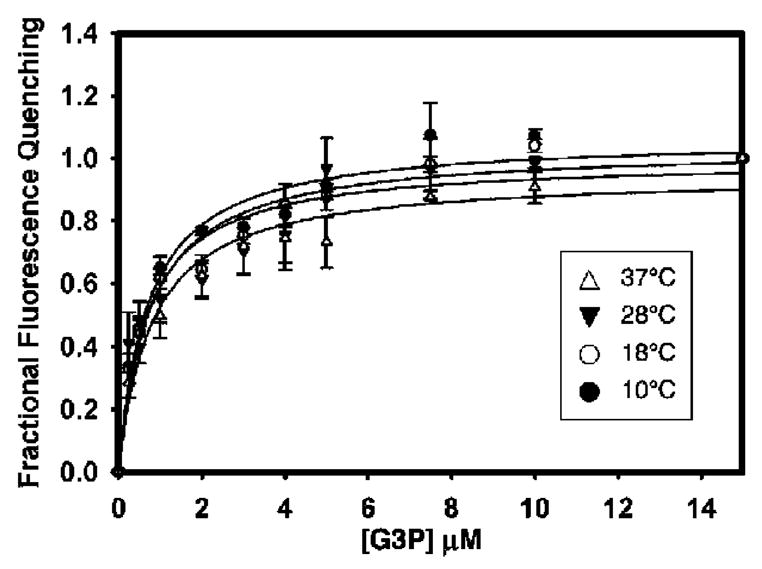
Measurement of G3P binding to GlpT at various temperatures using intrinsic tryptophan fluorescence quenching. G3P was titrated until fluorescence quenching was saturated (about 15 μM). The dissociation constant of G3P binding was found to be about 0.7 μM, and substrate binding did not show temperature dependence. Data points represent the mean ± SE of three separate measurements.
