Figure 6.
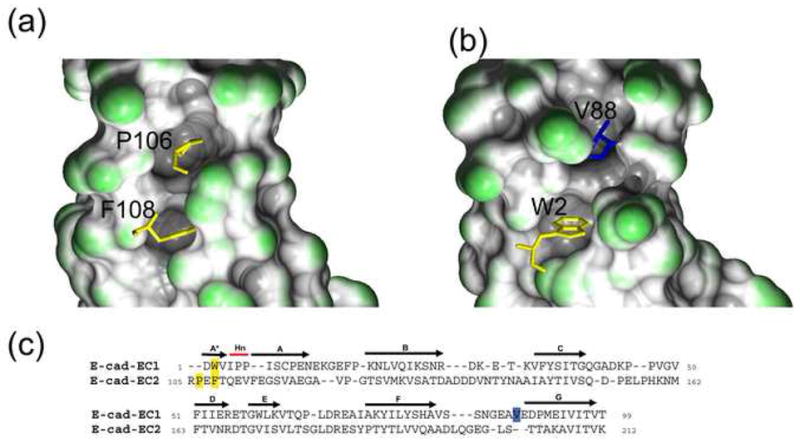
Pocket filling residues in EC2 and EC1. (a) In EC2, P106 of the A* strand PxF motif fills a pocket in the molecule. This interaction is shown with the PxF motif in stick representation and the molecular surface of the pocket drawn. The A* and A strand residues were not included in building the molecular surface. (b) In EC1, the equivalent pocket is filled by a residue of the G strand, V88. The A*, A strand, and A87-V88 residues were not included in building the molecular surface so as to highlight the filled pocket. (c) Structure-based sequence alignment of E-cadherin EC1 and E-cadherin EC2 (PDB 1FF5). N-terminal residues – P106 and F108 in EC2 and W2 in EC1 – are indicated in yellow. V88, in the EC1 G strand, is in blue.
