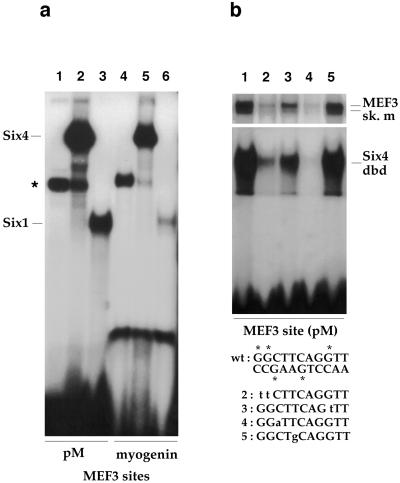
Figure 2.
Six1 and Six4 are able to bind the myogenin and aldolase A MEF3 motifs. (a) GMSAs performed with recombinant full-size Six4 (from in vitro T7-synthetized Six4 mRNAs translated in a rabbit reticulocyte lysate) and Six1 proteins (recombinant proteins purified after production in bacteria, see Materials and Methods). Mock lysate (lanes 1 and 4), Six4 translation products (lanes 2 and 5), or Six1 recombinant proteins (lanes 3 and 6) were incubated with labeled MEF3 sites from aldolase A (pM) or myogenin genes. ∗ indicates a nonspecific lysate binding activity. (b) GMSAs were performed with aldolase A MEF3 site (pM) and with either skeletal muscle (sk.m) nuclear extracts (Upper) or recombinant Six4 protein [corresponding to amino acids 1–240 of the AREC protein encompassing the DNA-binding domain (dbd) defined in ref. 15]. In lanes 2–5, 30 ng of double-stranded MEF3 site mutated in different nucleotides was added as competitor (lane 1, no competitor). ∗ indicate the G residues whose methylation inhibits protein binding in a dimethyl sulfate (DMS) interference assay using muscle nuclear extracts (not shown). The mutations were in the bases defined by DMS interference on the MEF3 complex, and the mutated MEF3 sequence site is indicated with mutated bases in small letters. Note that mutation of the T (mut5) in the sequence TCAGG completely abolished the competition, thus showing that this nucleotide is absolutely required for the binding of Six protein to the MEF3 site.