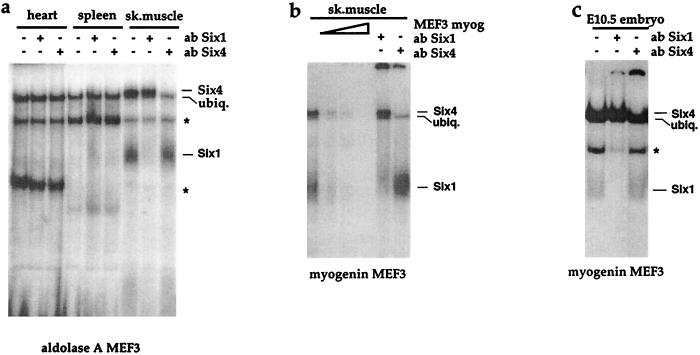
Figure 3.
Six1 and Six4 are the proteins that form the MEF3 muscle-specific binding activities. (a) GMSAs performed with adult nuclear extracts from different tissues on the MEF3 site of aldolase A. Five micrograms of nuclear extracts from heart, spleen, and skeletal muscles from the limb (sk.muscle) were incubated on ice with 0.3 ng of labeled double-stranded MEF3 site (pM). Six1 Ab or Six4 Ab were added subsequently thereafter, and the incubation mix then was kept on ice for 5 min. ∗ indicate nonspecific DNA-protein complexes (faintly competed by an excess of double-stranded MEF3 site). MEF3-binding activity comprises a ubiquitous complex (ubiq. MEF3) detected in each nuclear extract (including liver, not shown) and two muscle-specific complexes that did not form in the presence of anti-Six1 and anti-Six4 sera, respectively. Preimmune sera were not able to displace these complexes (not shown). In contrast, Abs against both Six1 and Six4 abolished the fast and slow migrating bands of the MEF3 DNA-protein complexes, respectively. (b) GMSAs performed with adult skeletal muscle (sk.muscle) nuclear extracts in the presence of the myogenin MEF3 site. Five micrograms of nuclear extracts were incubated on ice with 0.3 ng of labeled double-stranded myogenin MEF3 site. Increasing amounts (5, 15, or 50 ng) of myogenin MEF3 site was added in the reaction mix, as competitor. Abs against Six1 (Six1 Ab) or Six4 (Six4 Ab) were added subsequently, and the reaction mix then was kept on ice for 5 min. (c) GMSAs performed with protein extracts from embryonic trunks at E10.5 in the presence of the myogenin MEF3 site. Twenty micrograms of E10.5 protein extracts were incubated on ice with 0.3 ng of labeled double-stranded myogenin MEF3 site. Abs against Six1 (Six1 Ab) or Six4 (Six4 Ab) were added subsequently, and the reaction mix then was kept on ice for 5 min. ∗ indicates a nonspecific complex, which is not competed by an excess of MEF3 oligonucleotide and is not reproducibly displaced by Six1ab. ubiq., ubiquitous complex.