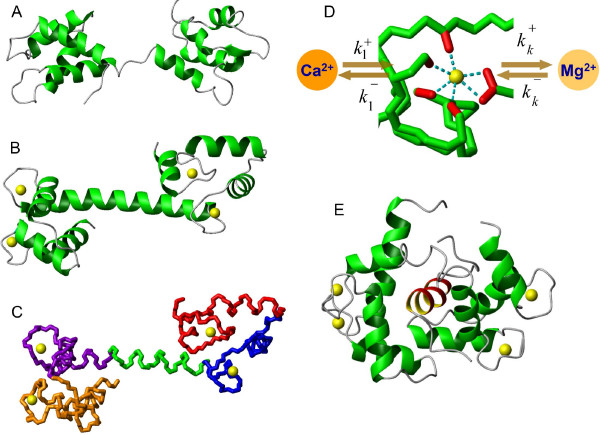
Figure 1.
The analysis of the CaM structures in apo and Ca2+ bound conformations as well as in complex with the target PKII molecule. The apo (1DMO [18]) (A) and Ca2+ bound (1CLL [19]) (B) structures of the CaM protein illustrate Ca2+ induced conformational transitions. (C) The backbone representation of the calcium bound form (1CLL [19]) highlights the pairwise proximity of the (red and blue, violet and brown) Ca2+ binding EF hand domains and at the same time raises doubts about overall cooperativity between binding sites. (D) Structure of the Ca2+ loop on the N-terminal of the CaM protein (1CLL [19]). The dotted line shows the interaction between oxygen atoms on the sidechains of Asp20, Asp22, Asp24, Thr26, Glu31 residues and Ca2+ or Mg2+ ions. (E) The ribbon diagram of the Ca2+-CaM in complex with the target peptide of the protein kinase kinase (1CKK [22]).