Abstract
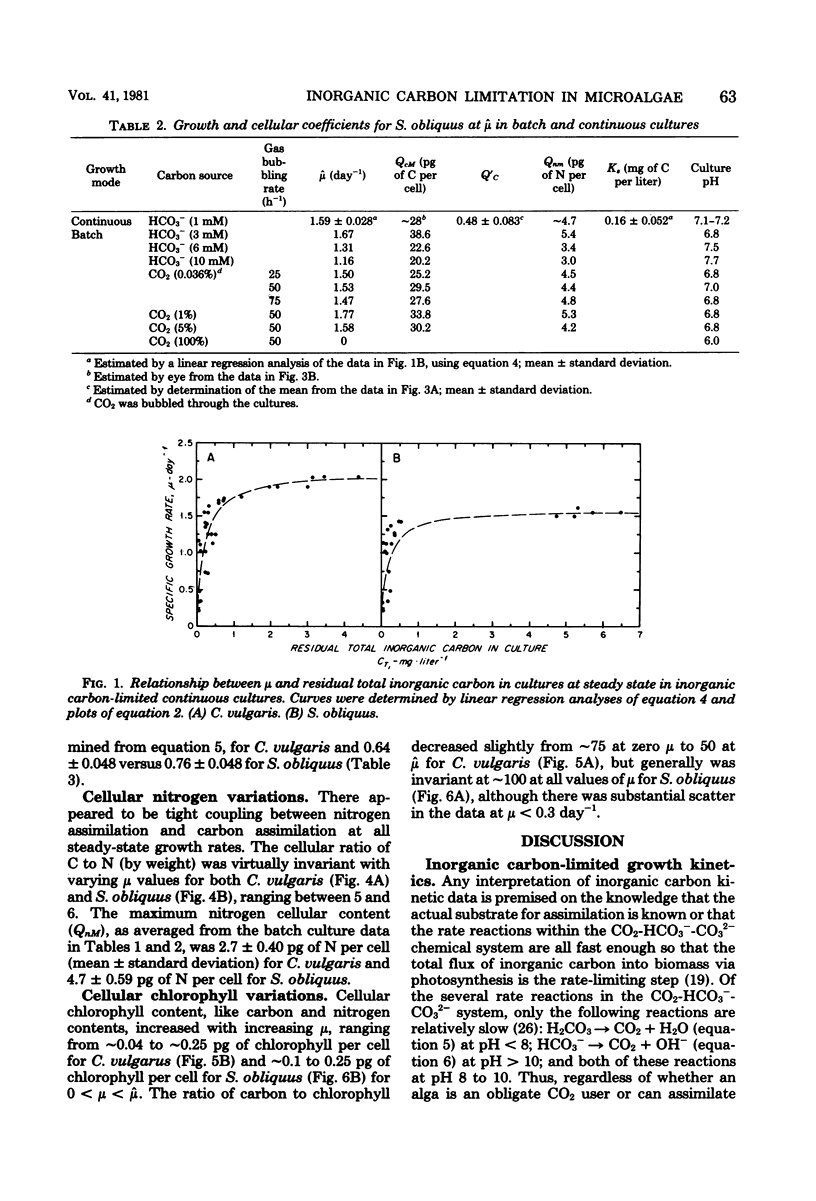
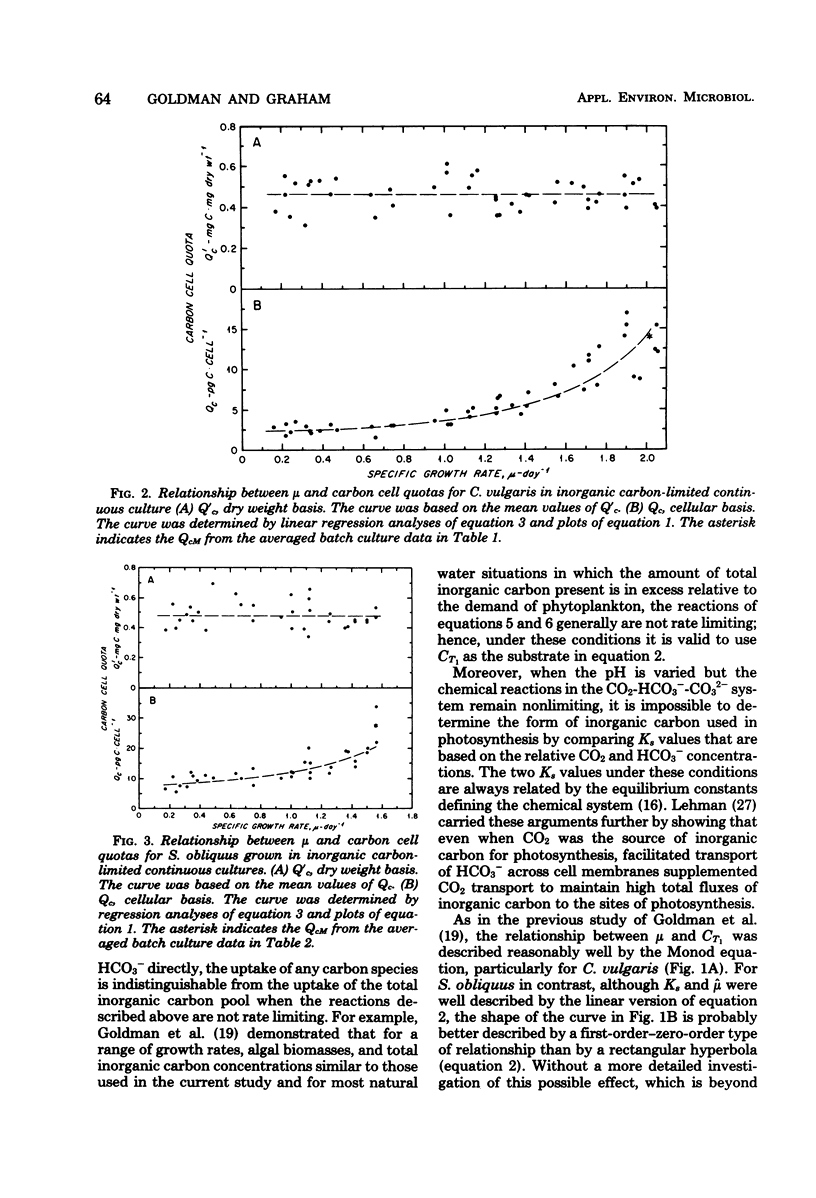
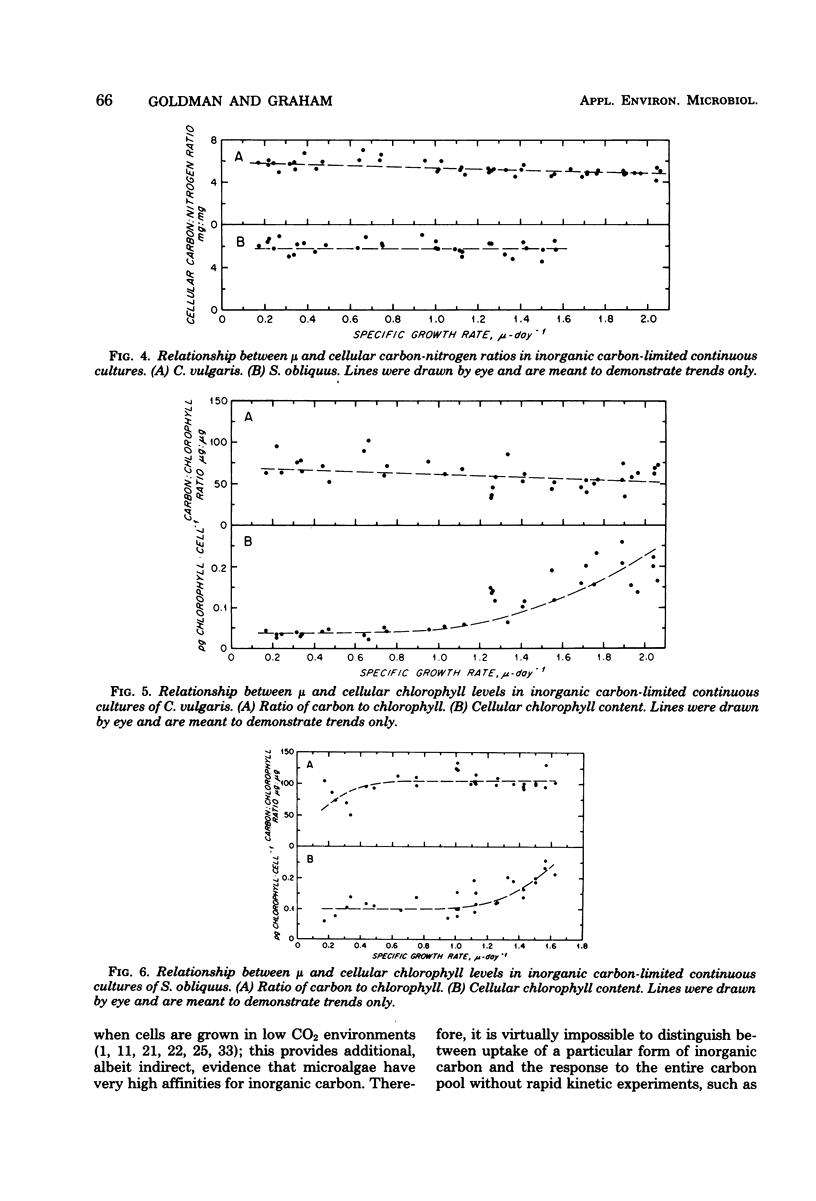
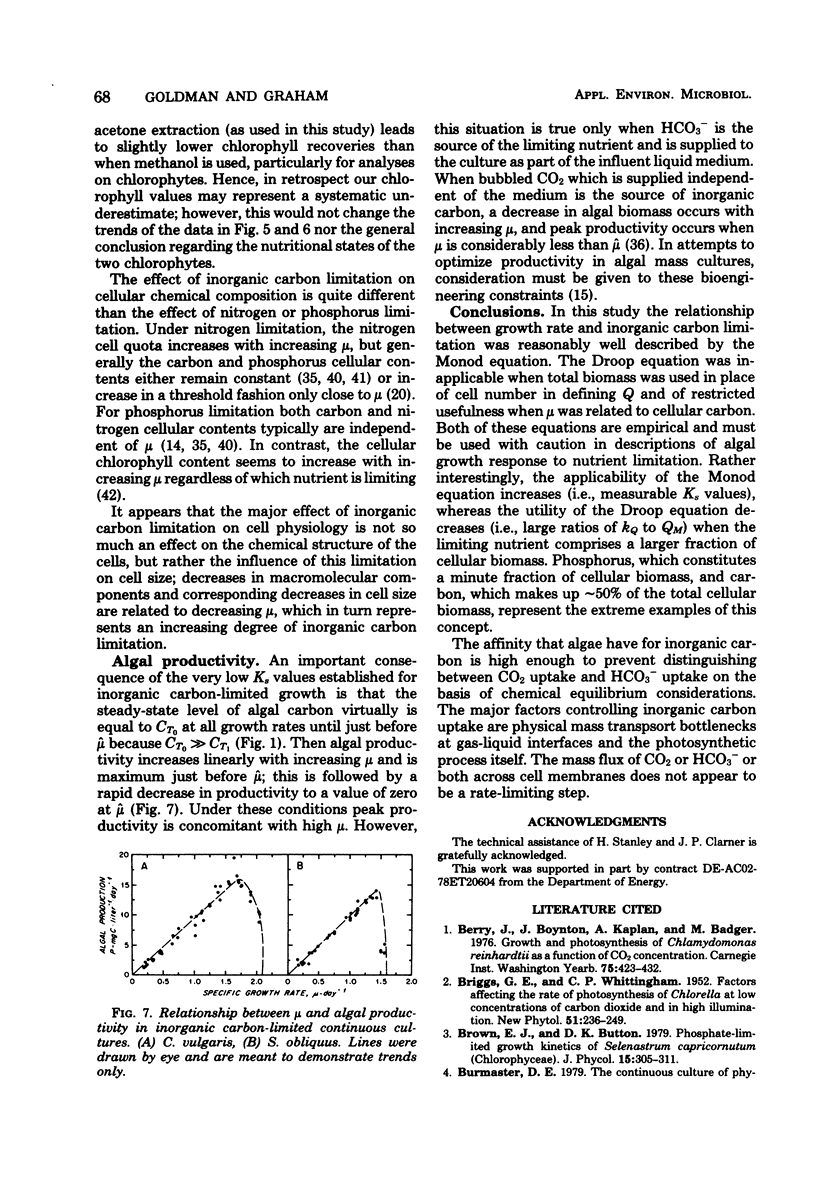
Two freshwater chlorophytes, Chlorella vulgaris and Scenedesmus obliquus, were grown in inorganic carbon-limited continuous cultures in which HCO3− was the sole source of inorganic carbon. The response of the steady-state growth rate to the external total inorganic carbon concentration was reasonably well described by the Monod equation; however, the response to the internal nutrient concentration was only moderately well represented by the Droop equation when the internal carbon concentration was defined on a cellular basis. The Droop equation was totally inapplicable when total biomass (dry weight) was used to define internal carbon because the ratio of carbon to dry weight did not vary over the entire growth rate spectrum. In batch cultures, maximum growth rates were achieved at the CO2 levels present in atmospheric air and at HCO3− concentrations of 3 mM. No growth was observed at 100% CO2. Both nitrogen uptake and chlorophyll synthesis were tightly coupled to carbon assimilation, as indicated by the constant C/N and C/chlorophyll ratios found at all growth rates. The main influence of inorganic carbon limitation appears to be not on the chemical structure of the biomass, but rather on cell size; higher steady-state growth rates lead to bigger cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Emerson R., Green L. EFFECT OF HYDROGEN-ION CONCENTRATION ON CHLORELLA PHOTOSYNTHESIS. Plant Physiol. 1938 Jan;13(1):157–168. doi: 10.1104/pp.13.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUILLARD R. R., RYTHER J. H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol. 1962 Apr;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Goldman J. C., Peavey D. G. Steady-State Growth and Chemical Composition of the Marine Chlorophyte Dunaliella tertiolecta in Nitrogen-Limited Continuous Cultures. Appl Environ Microbiol. 1979 Nov;38(5):894–901. doi: 10.1128/aem.38.5.894-901.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D., Reed M. L. Carbonic anhydrase and the regulation of photosynthesis. Nat New Biol. 1971 May 19;231(20):81–83. doi: 10.1038/newbio231081a0. [DOI] [PubMed] [Google Scholar]
- Myers J. THE GROWTH OF CHLORELLA PYRENOIDOSA UNDER VARIOUS CULTURE CONDITIONS. Plant Physiol. 1944 Oct;19(4):579–589. doi: 10.1104/pp.19.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIPES W. O. Carbon dioxide-limited growth of Chlorella in continuous cultures. Appl Microbiol. 1962 Jul;10:281–288. doi: 10.1128/am.10.4.281-288.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOROKIN C. Inhibition of cell division by carbon dioxide. Nature. 1962 May 5;194:496–497. doi: 10.1038/194496a0. [DOI] [PubMed] [Google Scholar]


