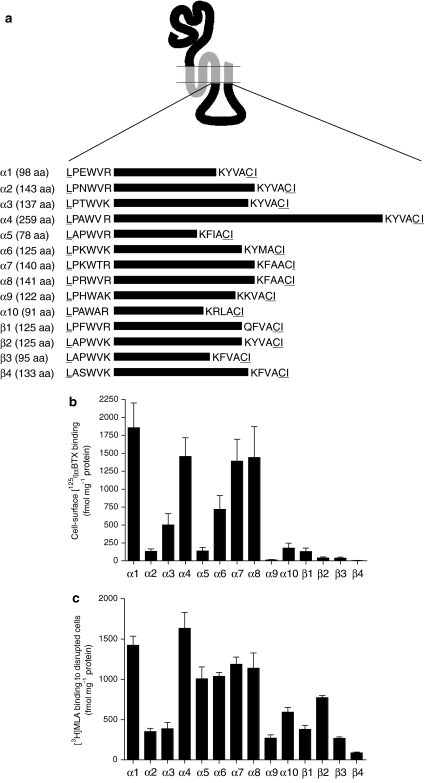
Figure 1.
Intracellular (M3–M4 loop) chimaeras. (a) A series of subunit chimaeras was constructed containing the intracellular M3–M4 loop domain from all vertebrate nAChR α and β subunits (α1–α10 and β1–β4). All subunit chimaeras contained a common extracellular domain (from the nAChR α7 subunit) and common transmembrane domains (from the 5-HT3A subunit). Horizontal lines represent the approximate relative length of the various intracellular loop domains of each subunit. The exact number of amino acids (aa) within each of these subunit domains is indicated next to the subunit name. To illustrate more clearly the location of the loop domains examined, the six N- and C-terminal amino acids of each domain are indicated. Amino acids that were altered by the introduction of NotI and BstZ17I restriction enzyme sites are underlined. (b) Cell-surface [125I]αBTX binding to loop chimaeras expressed in human tsA201 cells. (c) Binding of [3H]MLA to loop chimaeras determined with disrupted tsA201 cells. Data in (b and c) are presented as fmol per mg protein and are means of 3–7 independent experiments, each performed in triplicate. Error bars represent s.e.mean. αBTX, α-bungarotoxin; MLA, methyllycaconitine.