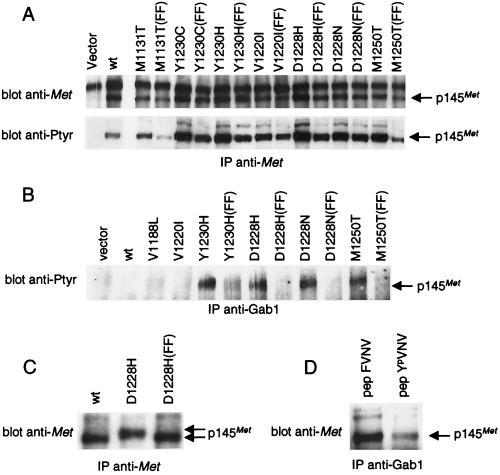
Figure 3.
METPRC mutants are constitutively coupled to signal transducers via two tyrosines located in the tail of the receptor. (A) NIH 3T3 fibroblasts were transfected with either wild-type or PRC-mutated MET receptor mutants to generate stable cell lines. FF indicates MET-receptor variants in which tyrosines Y1349 and Y1356 were converted into phenylalanines. The amount of Met proteins and the level of phosphorylation was evaluated by immunoprecipitation with anti-Met antibodies followed by Western blotting with anti-Met or anti-phosphotyrosine (pTyr) antibodies, respectively. (B) Lysates of the same cells were immunoprecipitated with anti-Gab1 antibodies and analyzed by Western immunoblotting with anti-pTyr antibodies. The identity of the phosphorylated protein coimmunoprecipitated with Gab1 was confirmed by reprobing the same blot with an anti-Met antibody (data not shown). (C) Lysates of COS cells expressing either the wild-type (wt), the D1228H mutant, or its double phenylalanine counterpart D1228H (FF) were immunoprecipitated and analyzed by Western blotting with anti-Met antibodies. The gap between the arrows indicates the electrophoretic shift caused by differences in phosphorylation. (D) Lysates of NIH 3T3 cells expressing the D1228H mutant were incubated with the YpVNV phosphopeptide corresponding to the receptor’s docking site and immunoprecipitated with anti-Gab1 antibodies. The amount of associated receptor was determined by Western blot with anti-Met antibodies. The nonphosphorylated phenylalanine peptide analogue (FVNV) was used as a negative control. The arrows indicate the position of the 145-kDa MET receptor β-chain (p145Met).