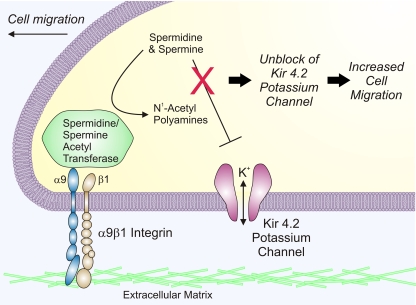
Fig. 1.
Model of enhanced cell migration mediated by α9β1 integrins, spermidine/spermine N1-acetyltransferase (SSAT), and inward rectifier potassium channels. The leading edge of a migrating cell is depicted. Engagement of the extracellular domain of α9β1 integrins with specific ligands in the extracellular matrix initiates an increase in cell migration. This process requires SSAT, a polyamine catabolic enzyme that binds to the α9 integrin cytoplasmic tail. SSAT catalyzes the acetylation of the higher-order polyamines spermine (+4 valence) and spermidine (+3 valence), producing acetylated polyamines with a lower charge that may be further degraded or excreted. Inward rectifier Kir 4.2 potassium channels also are required for α9β1 integrin-mediated cell migration. Normally, outward current through Kir 4.2 potassium channels would be blocked by voltage-dependent binding of spermine and spermidine in the channel pore, but acetylation of these polyamines is proposed to relieve the block, allowing increased permeation of K+ ions through the channel. The resulting increase in K+ channel activity, possibly acting through changes in local ion fluxes or membrane potential, or by other downstream channels or effectors, is proposed to enhance cell migration.