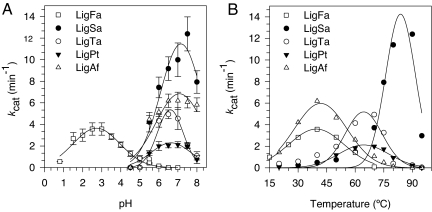
Fig. 1.
Recombinant LigFa has a uniquely low pH optimum. Activity vs. pH assays were performed by using a standard fluorimetric assay based on the ligation of a 5′-phosphorylated 35-meric and 5′-fluorescein-labeled 25-meric oligonucleotides annealed at the complementary 70-mer, as described in Materials and Methods, at the optimal temperature 40°C (LigFa and LigAf), 70°C (for LigTa and LigPt), or 80°C (for LigSa) in reaction buffers supplemented with 20 mM MgCl2 and 2 mM ATP, except for LigFa, where MgCl2 was not added. Plot of kcat against pH (A) and temperature (B) obtained by Eadie–Hofstee linearization for LigFa and DNA ligases from other acidophiles. Buffers (100 mM) used were sodium citrate for pH 0.5–3.0, sodium acetate for pH 4.0–5.0 and Mes for pH 6.0–7.0. All data were calculated from three independent assays ± SD and are not fitted to any model.