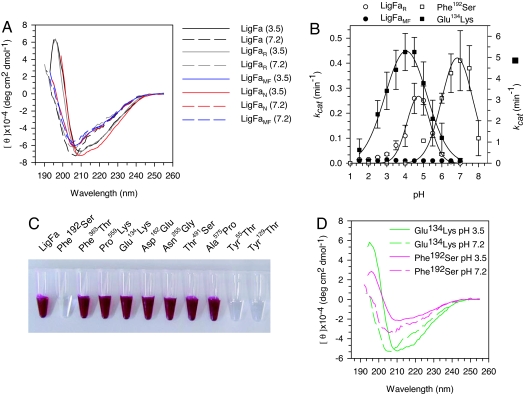
Fig. 3.
Properties of LigFa variants. (A) pH dependence of CD spectra of LigFa, reduced/ferrous iron-containing enzyme, LigFaR, and metal-free enzyme, LigFaMF. CD spectra of LigFa variants at acidic and neutral pHs were acquired as described in SI Text. (B) Influence of iron status and single point mutations on LigFa ligation activity (kcat) at each of different pHs. Iron variants were prepared and analyzed as described in SI Text. Assay conditions and buffers were as in Fig. 1. All data were calculated from three independent assays ± SD at 40°C and are not fitted to any model. Note the scale differences on the y axes (left axis: LigFaR, LigFaMF, and Phe192Ser; right, Glu134Lys). (C) Color phenotypes of LigFa variants (10 mg/ml) at pH 3.0. (D) CD spectra of Glu134→Lys134 and Phe192→Ser192 LigFa mutants at acidic and neutral pHs. Spectra were acquired as described in SI Text.