Abstract
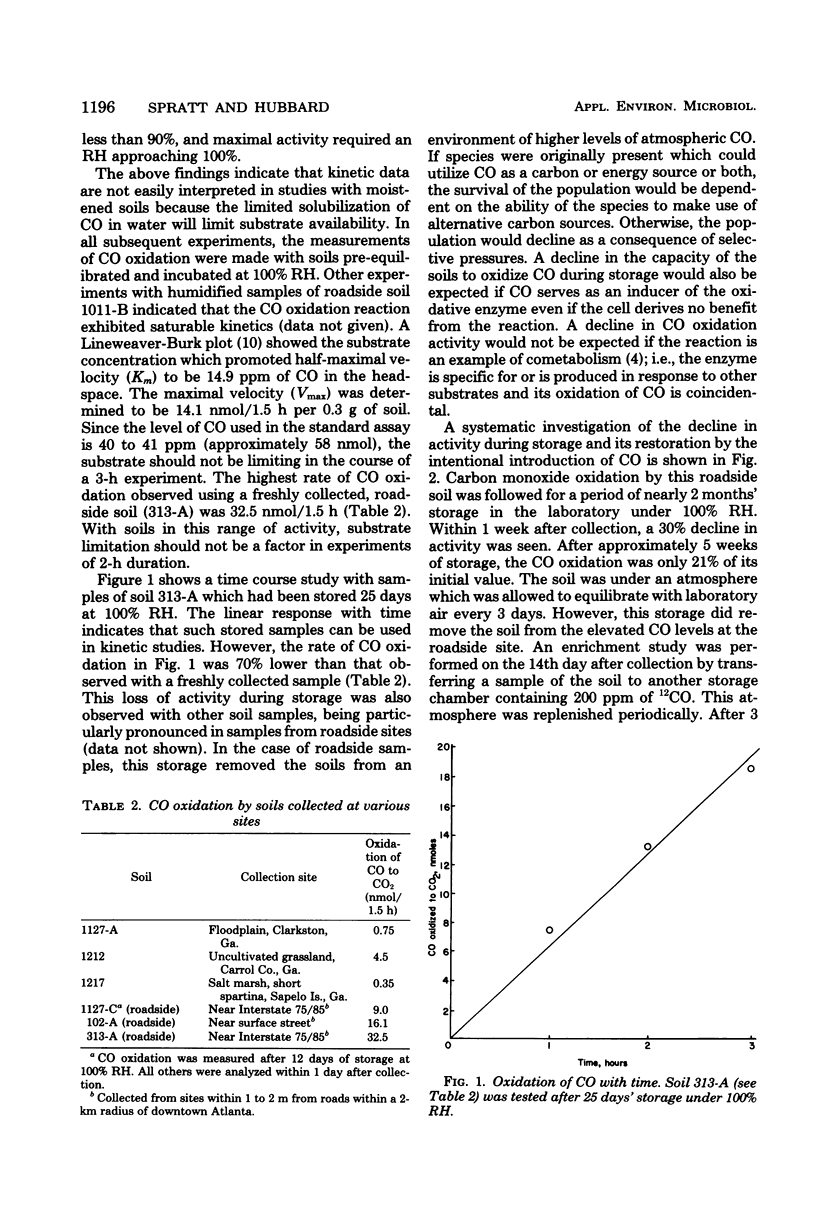
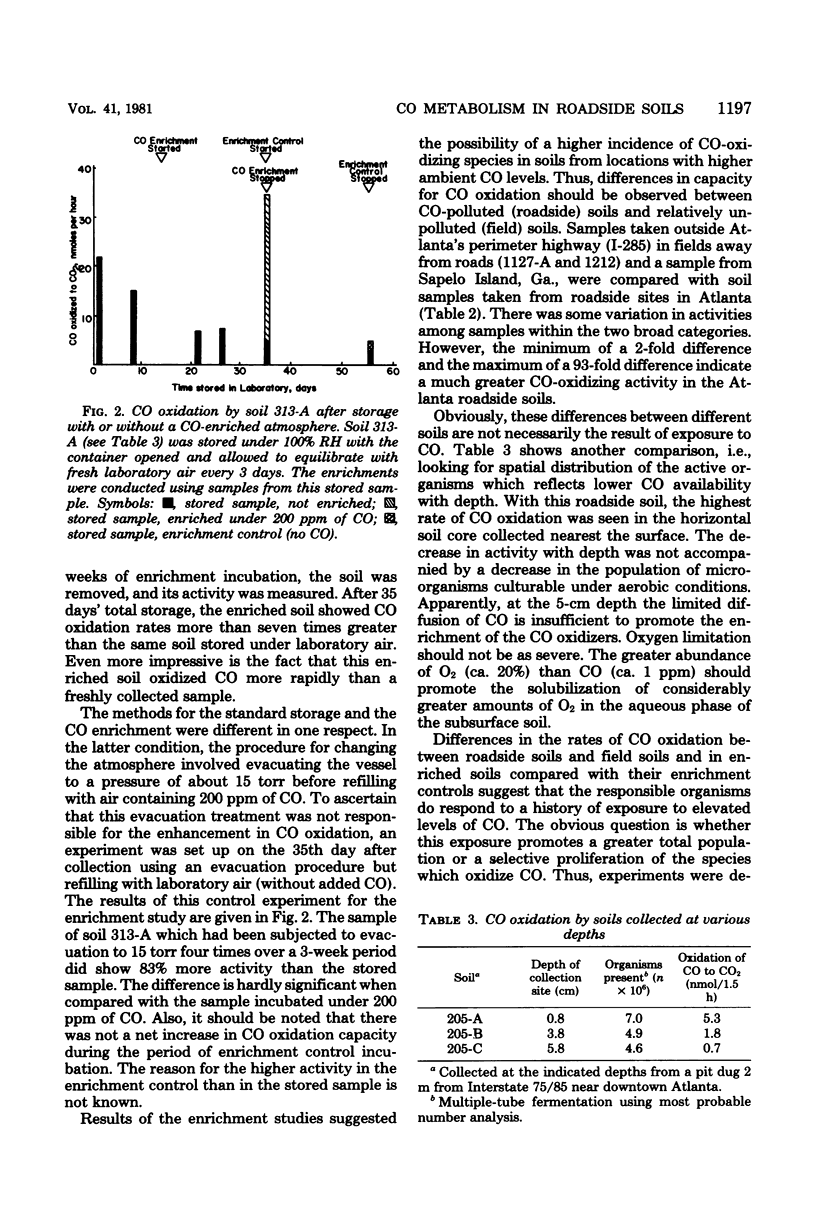
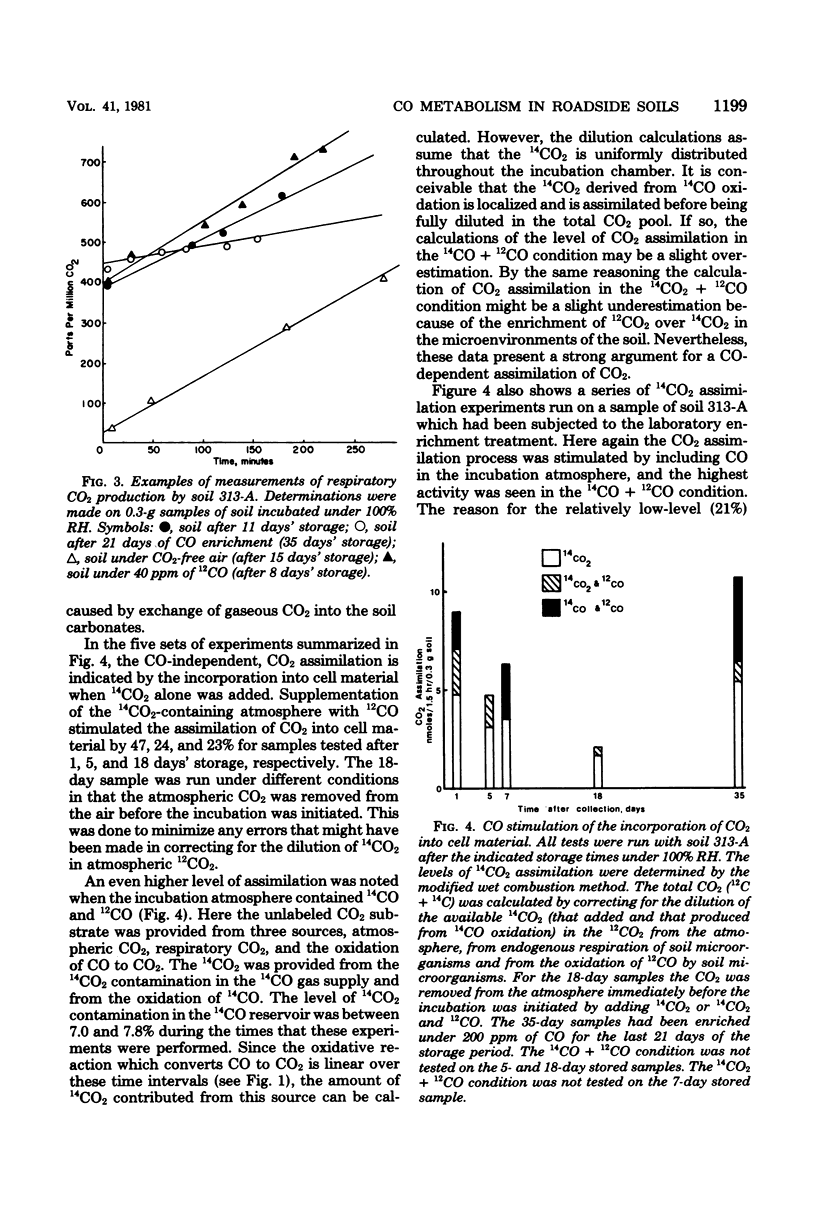
Air-dried soils which were equilibrated under relative humidities greater than 93% or moistened with liquid water showed marked increases in their capacities to oxidize CO to CO2. Liquid water addition in excess of saturation resulted in lower CO oxidation rates, reflecting the limited diffusion of CO through the aqueous phase. After 35 days' storage under 100% relative humidity, the capacity for CO oxidation decreased to 21% of the value observed with a freshly collected sample. Incubation of this stored soil under an atmosphere containing 200 ppm of CO (250 mg/m3) for 21 days resulted in a sevenfold increase in CO oxidation. A correlation was noted between the CO oxidative activity and the history of previous exposure of soils to high ambient levels of CO. The organisms responsible for CO oxidation apparently comprise a small fraction of the microbial population in the soils. With a roadside soil the oxidation of CO provided the driving force for the assimilation of CO2. The stoichiometry of the oxidative and assimilatory reactions in soil was in the range of values reported from laboratory studies with CO chemoautotrophs (carboxydobacteria). It is proposed that the population and activity of CO-oxidizing microorganisms increase in response to increasing levels of CO in the environment.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomew G. W., Alexander M. Microbial metabolism of carbon monoxide in culture and in soil. Appl Environ Microbiol. 1979 May;37(5):932–937. doi: 10.1128/aem.37.5.932-937.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath R. S. Microbial co-metabolism and the degradation of organic compounds in nature. Bacteriol Rev. 1972 Jun;36(2):146–155. doi: 10.1128/br.36.2.146-155.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. S. Laboratory simulations of the pyrolytic release experiments: an interim report. J Mol Evol. 1979 Dec;14(1-3):211–221. doi: 10.1007/BF01732379. [DOI] [PubMed] [Google Scholar]
- Hubbard J. S., Rinehart C. A., Baker R. A. Energy coupling in the active transport of amino acids by bacteriohodopsin-containing cells of Halobacterium holobium. J Bacteriol. 1976 Jan;125(1):181–190. doi: 10.1128/jb.125.1.181-190.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman R. E., Ingersoll R. B., Levy E. A. Soil: a natural sink for carbon monoxide. Science. 1971 Jun 18;172(3989):1229–1231. doi: 10.1126/science.172.3989.1229. [DOI] [PubMed] [Google Scholar]
- Paul E. A., Johnson R. L. Microscopic counting and adenosine 5'-triphosphate measurement in determining microbial growth in soils. Appl Environ Microbiol. 1977 Sep;34(3):263–269. doi: 10.1128/aem.34.3.263-269.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. W., Fliermans C. B., Brock T. D. Technique for measuring 14 CO 2 uptake by soil microorganisms in situ. Appl Microbiol. 1972 Mar;23(3):595–600. doi: 10.1128/am.23.3.595-600.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


