Abstract
The popularity of testosterone among drug users is due to its powerful effects on muscle strength and mass. Important mechanisms behind the myotrophic effects of testosterone were uncovered both in athletes using steroids for several years and in short-term controlled studies. Both long-term and short-term steroid usage accentuates the degree of fibre hypertrophy in human skeletal muscle by enhancing protein synthesis. A mechanism by which testosterone facilitates the hypertrophy of muscle fibres is the activation of satellite cells and the promotion of myonuclear accretion when existing myonuclei become unable to sustain further enhancement of protein synthesis. Interestingly, long-term steroid usage also enhances the frequency of fibres with centrally located myonuclei, which implies the occurrence of a high regenerative activity. Under the action of testosterone, some daughter cells generated by satellite cell proliferation may escape differentiation and return to quiescence, which help to replenish the satellite cell reserve pool. However, whether long-term steroid usage induces adverse effects of satellite cells remains unknown. Testosterone might also favour the commitment of pluripotent precursor cells into myotubes and inhibit adipogenic differentiation. The effects of testosterone on skeletal muscle are thought to be mediated via androgen receptors expressed in myonuclei and satellite cells. Some evidence also suggests the existence of an androgen-receptor-independent pathway. Clearly, testosterone abuse is associated with an intense recruitment of multiple myogenic pathways. This provides an unfair advantage over non-drug users. The long-term consequences on the regenerative capacity of skeletal muscle are unknown.
Keywords: satellite cells, anabolic steroids, exercise training, androgen receptor, hypertrophy, hyperplasia, muscle fibre, regeneration, myonuclei, protein synthesis
Introduction
The use of testosterone and related steroids is a widespread phenomenon among top athletes, amateurs, school-age children and a large part of the population who simply desire to improve their appearance. The popularity of testosterone and related steroids among drug users is due to the powerful effects of these substances on muscle strength and mass. Recent reports have uncovered important cellular and molecular mechanisms behind the myotrophic action of anabolic steroids. The effects of testosterone might be mediated via several myogenic pathways. This review starts with a brief description of the reasons for testosterone usage and the methods used in the detection of its abuse. The main focus is the description of the cellular and structural changes observed in human skeletal muscle in response to testosterone administration. Important cellular and molecular pathways by which testosterone might exert its action on skeletal muscle are discussed.
Reasons for testosterone abuse and side effects
Testosterone is a 19-carbon steroid with powerful androgenic and anabolic effects. Testosterone is primarily produced by the Leydig cells in the testes and a small quantity comes from the adrenal cortex and the peripheral conversion of androstenedione. The anabolic action of testosterone and related steroids on skeletal muscle is the reason for their popularity among drug users. Anabolic androgenic steroids are taken orally, by intramuscular injection, and as gels and creams. These drugs are used to increase lean body mass, to decrease fat mass, to enhance performance, to sustain intensive training periods and, finally, to improve the appearance (Yesalis, 1993; Hartgens and Kuipers, 2004). Through case reports, androgenic–anabolic drugs have been associated with a wide range of adverse effects: deleterious changes in risk factors associated with cardiovascular disease, alterations in liver structure and function, and in the reproductive system and changes in behaviour (Wilson, 1988; Yesalis, 1993; Hartgens and Kuipers, 2004). In this respect, treatment of human neuroblastoma cells with high doses of testosterone decreases cell viability by activation of cell death programmes indicating possible long-term effects of drug abuse on brain function (Estrada et al., 2006). Athletes often use doses that are far beyond those used in controlled studies, which implies that serious adverse effects of drug abuse may be under-recognized and more pronounced than what is currently described in scientific studies (Hartgens and Kuipers, 2004).
Detection of testosterone abuse
The fight against doping was initiated by the International Olympic Committee in the 1960s and androgenic–anabolic steroids were added on the list of banned substances in 1976. Doping by means of testosterone is difficult to uncover due to the fact that the hormone is also produced endogenously. Therefore, the critical issue in doping control is to establish the origin of testosterone found in human urine. Doping by testosterone can be indirectly tested using the urinary testosterone/epitestosterone (T/E) ratio (WADA, 2004). In normal healthy individuals, testosterone and epitestosterone are produced in a ratio of 1:1. Therefore, it is assumed that the urinary T/E ratio increases in athletes taking exogenous testosterone. If an athlete has a T/E of more than 4:1, the sample is submitted to GC-C-IRMS (gas chromatography–combustion–isotope ratio mass spectrometry) for determination of the 13C/12C ratio (WADA, 2004). This is because exogenous compounds contain less 13C than their endogenous homologue (Shackleton et al., 1997; Aguilera et al., 2001). However, if GC-C-IRMS does not conclusively indicate exogenous administration despite a T/E>4.0, longitudinal monitoring of the T/E–time profile is required. In this respect, the T/E ratio is characterized by a larger inter- than intra-individual variability (Sottas et al., 2007), and a T/E ratio in the range of 4:1 can be found even in individuals not taken testosterone. Therefore, a twofold increase of the T/E ratio might go undetected. For this reason, it is suggested that a population-based T/E reference is not sensitive to individual variations. According to Sottas et al. (2007), the problem can be statistically defined as the detection of an outlier out of series of individual test results using a Bayesian analysis of T/E ratio, that is, comparing a value to both a population-based reference range and a subject-based reference range.
Testosterone, muscle strength and mass
A main reason behind the popularity of testosterone among drug users is its effects on athletic performance and on muscle size. Suppression of endogenous testosterone production in young men by a gonadotropin-releasing hormone (GnRH) analogue resulted in marked decreases in the rates of whole-body protein synthesis, in muscle strength and in fat oxidation together with an increased adiposity (Mauras et al., 1998). Manipulation of the circulating testosterone levels by simultaneous treatment with GnRH analogues and exogenous testosterone showed the existence of a positive relationship between testosterone concentration and fat-free mass, muscle size and strength (Bhasin et al., 2001). Moreover, a randomized placebo-controlled and double-blinded intervention showed that the physiological response to a period of 12 weeks strength training is attenuated in a group of subjects receiving a GnRH analogue once every 4th week (Kvorning et al., 2006). The attenuation of the response to strength training included a reduced increase in lean leg mass and no changes in maximum isometric knee extensor strength. However, it is important to note that in the same study, the progression of training load during the 12 weeks training period in the group treated with a GNRH analogue was similar to the placebo-treated group (Kvorning et al., 2006). Moreover, suppression of testosterone does not seem to blunt mRNA expression of some members of the myogenic regulatory factors (MyoD and myogenin), insulin-like growth factor-1(IGF-1), myostatin and androgen receptors (ARs; Kvorning et al., 2007). This elucidates the complexity of the regulation of the signalling pathways behind the hypertrophy of human skeletal muscle in response to resistance training.
Improvements in muscle strength have been observed in response to the administration of testosterone. The amplitude of the effects of testosterone on muscle strength depends upon the initial muscle strength of the subjects, the doses used and the period of administration. Accordingly, consistent strength gains occurred in young healthy individuals receiving testosterone enanthate (300 mg week−1) for 6 weeks (Friedl et al., 1991). Similarly, the administration of supraphysiological doses of testosterone (600 mg week−1) for 10 weeks in untrained and trained men produced a significant increase in muscle strength and in the cross-sectional area of the quadriceps (Bhasin et al., 1996). It was also shown that moderate doses of testosterone combined with weight training induced short-term changes in upper body strength and body composition (Giorgi et al., 1999). Data also suggest that the effects of testosterone administration on human skeletal muscle mass is dose dependent (Bhasin et al., 2001). Interestingly, the use of testosterone in conjunction with heavy resistance training seems to be associated with changes in muscle pennation angle and possibly fascicle length (Blazevich and Giorgi, 2001).
Testosterone and the hypertrophy of muscle fibres
Important mechanisms behind the strong myotrophic effects of testosterone were first uncovered in a population of high-level powerlifters who reported the use of testosterone (100–500 mg week−1) for a period of 9±3.3 years (Kadi et al., 1999; Kadi, 2000). Long-term administration of testosterone accentuates the degree of fibre hypertrophy in already well-trained powerlifters (Kadi et al., 1999; Kadi, 2000). Testosterone induces the hypertrophy of both type I and type II muscle fibres. Type II muscle fibres are the largest muscle fibres in powerlifters both in steroid users and non-users. However, there is evidence suggesting that the largest difference in muscle fibre size between steroid users and non-users is observed in slow type I muscle fibres (Kadi et al., 1999; Kadi, 2000; Eriksson et al., 2005). In the trapezius muscle of steroid users, the area of type I muscle fibres is 58% larger than in non-users, whereas the area of type II muscle fibres is 33% larger than in non-users (Kadi et al., 1999). The same tendency is observed in the vastus lateralis (Eriksson et al., 2005). Accordingly, it has been shown that type I muscle fibres are more sensitive to anabolic agents than type II muscle fibres (Hartgens et al., 1996). Subsequently, it has been shown that the administration of 300 and 600 mg testosterone induced an increase in the area of type I muscle fibres, whereas type II muscle fibres enlarge only after administration of 600 mg testosterone (Sinha-Hikim et al., 2002).
Testosterone, protein synthesis and myonuclear content
Enhanced contractile protein synthesis is an important mechanism by which testosterone can enhance the size of muscle fibres. Intramuscular injection of 200 mg testosterone enanthate in healthy individuals induced a significant twofold increase in net protein synthesis, whereas protein breakdown was unchanged (Ferrando et al., 1998). Testosterone did not affect inward amino-acid transport to muscle but increased re-utilization of intracellular amino acids in skeletal muscle (Ferrando et al., 1998).
Adult muscle fibres contain hundreds of myonuclei, where each myonucleus sustains the protein synthesis over a finite volume of cytoplasm, a concept called ‘nuclear domain' (Cheek, 1985). In this respect, significant enlargement of muscle fibres (36% increase in fibre cross-sectional area) is accompanied by a significant increase in the myonuclear number, whereas no alterations in the number of myonuclei are observed when the increase in fibre area does not exceed approximately 26% (Kadi, 2000; Kadi et al., 2004b, 2005). Therefore, it is suggested that existing myonuclei are able to support a certain level of fibre hypertrophy. However, when the transcriptional activity of existing myonuclei reaches its maximum, the enhancement of the number of myonuclei is thought to become involved in the enhancement of protein synthesis, a concept termed the ceiling theory (Kadi et al., 2004b, 2005; Petrella et al., 2006). This is further supported by the relationship between the cross-sectional area of muscle fibres and the number of myonuclei per fibre cross-section (Kadi, 2000; Kadi et al., 2006). In this respect, a mechanism by which testosterone facilitates the hypertrophy of muscle fibres seen in drug users is to promote myonuclear accretion (Kadi et al., 1999; Kadi, 2000; Sinha-Hikim et al., 2002). In high-level powerlifters, the mean number of myonuclei per fibre cross-section is significantly higher in steroid users compared with non-users, and myonuclear accretion is greater in type I fibres (+23%) compared with type II muscle fibres (+14%) (Kadi, 2000). This is in accordance with the larger hypertrophy of type I muscle fibres seen in steroid users.
Testosterone and centrally located myonuclei
In steroid-using powerlifters, the number of muscle fibres with internal myonuclei reaches 25% in trapezius muscle and 29% in vastus lateralis (Kadi et al., 1999; Eriksson et al., 2005). In non-steroid-using powerlifters, the number of fibres with internal myonuclei is 5% in trapezius and 9% in the vastus lateralis (Kadi et al., 1999; Eriksson et al., 2005). This indicates that testosterone is associated with a three- to fivefold increase in centrally located myonuclei in the vastus lateralis and trapezius, respectively. In steroid users, centrally located myonuclei are encountered in both type I and type II muscle fibres. In contrast, centrally located myonuclei in non-steroid users are mainly located in type II muscle fibres (Kadi et al., 1999). The presence of internal myonuclei is traditionally recognized as an indication of ongoing muscle regeneration. The activation of satellite cells can lead to proliferation and differentiation into new myotubes that might fuse with existing muscle fibres. During the fusion process, some myonuclei might be trapped in the central part of the resulting new and larger fibre. This physiological positioning of the myonuclei might be required for fibre growth when new myotubes fuse with the existing parent muscle fibre. Centrally located myonuclei might remain in their position for a period after the fusion and that would reduce the diffusion distance from a nucleus to the central part of the myofibre.
Testosterone and satellite cells
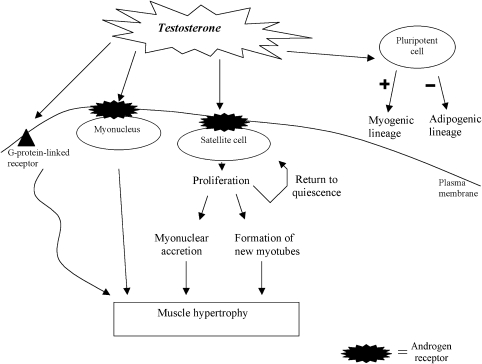
Satellite cells are located between the basal lamina and the plasma membrane of muscle fibres (Mauro, 1961). In human skeletal muscle, satellite cell content varies between muscles with different functional properties and between individuals with different physical activity levels and ages (Kadi et al., 2004a, 2005). In human vastus lateralis muscle of young adults, the number of satellite cells per fibre cross-section does not differ between type I and type II muscle fibres (Kadi et al., 2006). Existing myonuclei in adult muscle fibres are post mitotic, and muscle satellite cells are the major source for the addition of new myonuclei into the hypertrophying muscle fibre (Moss and Leblond, 1971). A variety of alterations in the surrounding environment of the satellite cell, including mechanical and growth factors and also hormonal signalling might regulate the activation and proliferation of satellite cells (Kadi, 2005; Kadi et al., 2005; Mackey et al., 2007). Satellite cells can proliferate and withdraw from differentiation to return to quiescence to replenish satellite cell pool, or to enter differentiation to provide new myonuclei or to generate new muscle fibres (Figure 1) (Kadi, 2000; Kadi et al., 2005). The mechanisms regulating the fate of daughter cells generated by satellite cell activation are currently not understood. Testosterone is able to stimulate the mitotic activity of satellite cells in myoblast culture systems (Powers and Florini, 1975). Furthermore, in response to the administration of testosterone, an increase in the number of PCNA+ (proliferating cell nuclear antigen-positive) satellite cells occurs in human skeletal muscle. The presence of satellite cells expressing the PCNA indicates that testosterone can promote the entry of satellite cells into the cell cycle (Sinha-Hikim et al., 2006). This highlights the role of satellite cells as mediators of the myotrophic action of testosterone on skeletal muscle. When satellite cells are forced to enter the cell cycle, some daughter cells escape differentiation and can return to quiescence, which ultimately lead to the generation of new satellite cells. In this respect, a significant increase in the number of satellite cells has been reported in men receiving testosterone (300 and 600 mg week−1) during 20 weeks (Sinha-Hikim et al., 2003). The number of satellite cells in skeletal muscle of powerlifters using anabolic steroids for a period of 9±3.3 years is higher than in healthy young men, but it remains similar to that seen in non-steroid-using powerlifters (Kadi, 2000). It might be hypothesized that short-term administration of steroids favours the generation of new satellite cells, whereas in long-term users the generation of new satellite cells might not be the main fate of the newly generated daughter cells. Alternatively, using steroids for many years attenuates their action on satellite cells.
Figure 1.
Mechanisms of testosterone action on skeletal muscle.
Muscle satellite cells are the stem cells of skeletal muscle. As such, they have the ability to maintain a ready source of muscle fibre precursors. Several mechanisms are proposed to explain the self-renewal of satellite cells in skeletal muscle. Asymmetric and/or symmetric cell divisions would lead to one or two daughter cells to become new satellite cells. Notch is a transmembrane receptor that can activate transcription factors involved in the regulation of cell fate. It has been proposed that the asymmetric expression of Numb (membrane-associated inhibitor of the Notch signalling) in dividing satellite cells in vitro might account for the occurrence of asymmetric cell divisions (Conboy and Rando, 2002). Numb-positive cells would enter differentiation, whereas Numb-negative cells would escape differentiation and become a satellite cell. It is hypothesized that Numb-negative cells would continue to proliferate and re-populate the satellite cell pool (Conboy and Rando, 2002). Thus, the interaction between Notch and its antagonist Numb might represent one pathway involved in the control of the fate of newly generated cells. In this respect, it is suggested that testosterone might influence the fate of newly generated daughter cells by enhancing the expression of the activated form of Notch, thus promoting cell proliferation and generation of new satellite cells (Sinha-Hikim et al., 2006).
Testosterone and the commitment of pluripotent precursor cells into myogenic lineage
It is suggested that the formation of new myotubes can also be achieved via the contribution of stem cells from sources other that satellite cells (Figure 1). However, the contribution of these muscle precursor cells in both physiological and supraphysiological adaptations of human skeletal muscle remains unclear. Nevertheless, it has been shown that mouse C3H 10T1/2 pluripotent mesenchymal cells (cells capable of differentiating into muscle, fat, cartilage and bone cells) treated with testosterone start to express specific myogenic markers (MyoD and myosin heavy chains) (Singh et al., 2003). This can be an indication of the ability of testosterone to recruit mesenchymal pluripotent cells into the myogenic lineage.
Testosterone and the adipogenic differentiation
Testosterone can also reduce body fat, as it is a potent regulator of lipolysis by influencing catecholamine signal transduction in fat cells (Arner, 2005) (Figure 1). It has been suggested that testosterone inhibits lipid uptake in adipocytes and stimulates lipolysis (De Pergola, 2000). Similarly, evidences suggest that testosterone can inhibit differentiation of adipocyte precursor cells (De Pergola, 2000). In agreement with these data, treatment of mouse C3H 10T1/2 pluripotent cells with testosterone inhibits adipogenic differentiation assessed by adipocyte counting and the expression of two adipogenic inhibitory factors (PPARδ2 (peroxisomal proliferator-activated receptor) and CCAAT/enhancer-binding protein α) (Singh et al., 2003).
Androgen receptors: mediators of testosterone effects
The hypertrophy of muscle fibres is a process under the complex control of several myogenic pathways (Figure 1). In this respect, the blockade of ARs by oxendolone, an AR antagonist, suppressed the hypertrophy of rat muscle fibres (Inoue et al., 1994). This experiment clearly elucidates the role played by ARs as potential mediators of the exercise-induced muscle fibre hypertrophy. Experiments also demonstrated substantial increases in the concentration of AR in response to exercise (Deschenes et al., 1994; Bamman et al., 2001). ARs belong to the super family of ligand-responsive transcription regulators. When androgenic hormones bind to the receptor, it becomes activated and the androgen-receptor complex is translocated to the hormone-responsive element within the nucleus. The binding to selective genes increases rates of transcription (Luke and Coffey, 1994). The number of binding sites per mg of protein is much lower in skeletal muscle than in the prostate in rats (Krieg, 1976). Two reports failed to identify a positive immunostaining in myonuclei of human muscle fibres (Ruizeveld de Winter et al., 1991; Janssen et al., 1994). The lack of staining using conventional immunohistochemical methods is probably due to the low level of AR in skeletal muscle. However, when immunohistochemistry is performed using a signal amplification technique, positive immunolabeling is observed in human vastus lateralis and trapezius muscles (Kadi, 2000; Kadi et al., 2000). Immunolabeling is also observed in capillary endothelium as well as in intramuscular nerve bundles (Kadi et al., 2000). Interestingly, not all myonuclei express AR and muscles of different sites may vary in AR content (Kadi et al., 2000). Quantification of AR-containing myonuclei per fibre cross-section revealed significant differences between two different muscles: in untrained subjects, the proportion of AR-containing myonuclei in the trapezius was nearly 60% higher than in the vastus lateralis (Kadi et al., 2000). These results are supported by data suggesting that androgen sensitivity may vary between different muscle groups (Kochakian and Tillotson, 1957; Wilson, 1988).
Self-administration of androgenic–anabolic steroids can alter the proportion of AR-containing myonuclei in human skeletal muscle. In the trapezius of steroid-using athletes, the myonuclear number and the percentage of AR-positive myonuclei are higher than in non-steroid users. In the vastus lateralis of steroid-using athletes, the number of myonuclei is higher but the percentage of AR-containing myonuclei is similar to non-steroid using athletes. (Kadi et al., 2000). Another study showed that AR expression is enhanced after 1 month of treatment with testosterone (Ferrando et al., 2002). However, when testosterone is administrated for 6 months AR expression returns to pretreatment levels (Ferrando et al., 2002). It has been suggested that the expression of AR can reach a steady state in response to the treatment paradigm (Ferrando et al., 2002). Altogether these results show the complexity of AR regulation in human skeletal muscle both under physiological and supraphysiological conditions.
Satellite cells express androgen receptors
The effect of testosterone on satellite cells is supported by the immunological detection of AR in porcine myogenic satellite cells in vitro (Doumit et al., 1996) (Figure 1). Furthermore, immunoreactive AR increased in response to testosterone treatment (Doumit et al., 1996). Thus, satellite cells are direct targets for testosterone action. Similar results were subsequently found in human muscle cells (Sinha-Hikim et al., 2004). The increase in androgen-binding sites might be important for the regulation of pathways involved in the control of satellite cell activity.
Androgen-receptor-independent pathway: mediators of testosterone effects
Recent studies suggest the existence of a rapid intracellular AR-independent mode of action for testosterone (Figure 1). This rapid non-genomic testosterone action may be exerted via increased intracellular Ca2+ concentration through the activation of a G-protein-linked receptor at the plasma membrane of myoblasts obtained from rat neonatal hind limbs (Estrada et al., 2003). This would result in an early but transient ERK1/2 (extracellular signal-regulated kinases) activation, which can potentially lead to the phosphorylation of transcription factors associated with cellular growth (Estrada et al., 2003). In the context of human skeletal muscle, the physiological significance of the rapid non-genomic action of testosterone remains unclear.
Conclusion
Testosterone has a powerful effect on human skeletal muscle. Data gathered on the muscular effects of testosterone clearly demonstrate that drug abuse is associated with an intense recruitment of multiple myogenic pathways. Clearly, testosterone administration in sports provides an unfair muscular advantage over non-drug users. The long-term consequences of the heavy recruitment of satellite cells on their proliferative potential and the regenerative capacity of skeletal muscle are unknown.
Abbreviations
- AR
androgen receptor
- GnRH
gonadotropin-releasing hormone
- PCNA
proliferating cell nuclear antigen
- T/E ratio
testosterone/epitestosterone ratio
Conflict of interest
The author states no conflict of interest.
References
- Aguilera R, Chapman TE, Starcevic B, Hatton CK, Catlin DH. Performance characteristics of a carbon isotope ratio method for detecting doping with testosterone based on urine diols: controls and athletes with elevated testosterone/epitestosterone ratios. Clin Chem. 2001;47:292–300. [PubMed] [Google Scholar]
- Arner P. Effects of testosterone on fat cell lipolysis. Species differences and possible role in polycystic ovarian syndrome. Biochimie. 2005;87:39–43. doi: 10.1016/j.biochi.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Bamman MM, Shipp JR, Jiang J, Gower BA, Hunter GR, Goodman A, et al. Mechanical load increases muscle IGF-I and androgen receptor mRNA concentrations in humans. Am J Physiol Endocrinol Metab. 2001;280:383–390. doi: 10.1152/ajpendo.2001.280.3.E383. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, et al. Testosterone dose–response relationships in healthy young men. Am J Physiol. 2001;281:1172–1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- Blazevich AJ, Giorgi A. Effect of testosterone administration and weight training on muscle architecture. Med Sci Sports Exerc. 2001;33:1688–1693. doi: 10.1097/00005768-200110000-00012. [DOI] [PubMed] [Google Scholar]
- Cheek DB. The control of cell mass and replication. The DNA unit—a personal 20-year study. Early Hum Dev. 1985;12:211–239. doi: 10.1016/0378-3782(85)90144-6. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- De Pergola G. The adipose tissue metabolism: role of testosterone and dehydroepiandrosterone. Int J Obes Relat Metab Disord. 2000;24 Suppl 2:S59–S63. doi: 10.1038/sj.ijo.0801280. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Maresh CM, Armstrong LE, Covault J, Kraemer WJ, Crivello JF. Endurance and resistance exercise induce muscle fiber type specific responses in androgen binding capacity. J Steroid Biochem Mol Biol. 1994;50:175–179. doi: 10.1016/0960-0760(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Doumit ME, Cook DR, Merkel RA. Testosterone up-regulates androgen receptors and decreases differentiation of porcine myogenic satellite cells in vitro. Endocrinology. 1996;137:1385–1394. doi: 10.1210/endo.137.4.8625915. [DOI] [PubMed] [Google Scholar]
- Eriksson A, Kadi F, Malm C, Thornell LE. Skeletal muscle morphology in power-lifters with and without anabolic steroids. Histochem Cell Biol. 2005;124:167–175. doi: 10.1007/s00418-005-0029-5. [DOI] [PubMed] [Google Scholar]
- Estrada M, Espinosa A, Müller M, Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144:3586–3597. doi: 10.1210/en.2002-0164. [DOI] [PubMed] [Google Scholar]
- Estrada M, Varshney A, Ehrlich BE. Elevated testosterone induces apoptosis in neuronal cells. J Biol Chem. 2006;281:25492–25501. doi: 10.1074/jbc.M603193200. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol. 2002;282:601–607. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Tipton KD, Doyle D, Phillips SM, Cortiella J, Wolfe RR. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol. 1998;275:864–871. doi: 10.1152/ajpendo.1998.275.5.E864. [DOI] [PubMed] [Google Scholar]
- Friedl KE, Dettori JR, Hannan CJ, Patience TH, Plymate SR. Comparison of the effects of high dose testosterone and 19-nortestosterone to a replacement dose of testosterone on strength and body composition in normal men. J Steroid Biochem Mol Biol. 1991;40:607–612. doi: 10.1016/0960-0760(91)90283-b. [DOI] [PubMed] [Google Scholar]
- Giorgi A, Weatherby RP, Murphy PW. Muscular strength, body composition and health responses to the use of testosterone enanthate: a double blind study. J Sci Med Sport. 1999;2:341–355. doi: 10.1016/s1440-2440(99)80007-3. [DOI] [PubMed] [Google Scholar]
- Hartgens F, Kuipers H. Effects of androgenic–anabolic steroids in athletes. Sports Med. 2004;34:513–554. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- Hartgens F, Kuipers H, Wijnen JA, Keizer HA. Body composition, cardiovascular risk factors and liver function in long-term androgenic–anabolic steroids using bodybuilders three months after drug withdrawal. Int J Sports Med. 1996;17:429–433. doi: 10.1055/s-2007-972873. [DOI] [PubMed] [Google Scholar]
- Inoue K, Yamasaki S, Fushiki T, Okada Y, Sugimoto E. Androgen receptor antagonist suppresses exercise-induced hypertrophy of skeletal muscle. Eur J Appl Physiol Occup Physiol. 1994;69:88–91. doi: 10.1007/BF00867933. [DOI] [PubMed] [Google Scholar]
- Janssen PJ, Brinkmann AO, Boersma WJ, Van der Kwast TH. Immunohistochemical detection of the androgen receptor with monoclonal antibody F39.4 in routinely processed, paraffin-embedded human tissues after microwave pre-treatment. J Histochem Cytochem. 1994;42:1169–1175. doi: 10.1177/42.8.8027537. [DOI] [PubMed] [Google Scholar]
- Kadi F. Adaptation of human skeletal muscle to training and anabolic steroids. Acta Physiol Scand. 2000;646:1–52. [PubMed] [Google Scholar]
- Kadi F.Hormonal and growth factor-related mechanisms involved in the adaptation of skeletal muscle to exercise The endocrine System in Sports and Exercise. Olympic Encyclopaedia of Sports Medicine 2005Blackwell: Massachusetts, USA; 306–318.In: Kraemer W, Rogol A (eds).vol. XI, Chapter 22. [Google Scholar]
- Kadi F, Bonnerud P, Eriksson A, Thornell LE. The expression of androgen receptors in human neck and limb muscles: effects of training and self-administration of androgenic–anabolic steroids. Histochem Cell Biol. 2000;113:25–29. doi: 10.1007/s004180050003. [DOI] [PubMed] [Google Scholar]
- Kadi F, Charifi N, Denis C, Lexell J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve. 2004a;29:120–127. doi: 10.1002/mus.10510. [DOI] [PubMed] [Google Scholar]
- Kadi F, Charifi N, Denis C, Lexell J, Andersen JL, Schjerling P, et al. The behaviour of satellite cells in response to exercise: what have we learned from human studies. Pflugers Arch. 2005;451:319–327. doi: 10.1007/s00424-005-1406-6. [DOI] [PubMed] [Google Scholar]
- Kadi F, Charifi N, Henriksson J. The number of satellite cells in slow and fast fibres from human vastus lateralis muscle. Histochem Cell Biol. 2006;126:83–87. doi: 10.1007/s00418-005-0102-0. [DOI] [PubMed] [Google Scholar]
- Kadi F, Eriksson A, Holmner S, Thornell L-E. Effects of anabolic steroids on the muscle cells of strength-trained athletes. Med Sci Sports Exerc. 1999;31:1528–1534. doi: 10.1097/00005768-199911000-00006. [DOI] [PubMed] [Google Scholar]
- Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR, et al. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol. 2004b;558:1005–1012. doi: 10.1113/jphysiol.2004.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochakian CD, Tillotson C. Influence of several C19-steroids on the growth of individual muscles of the guinea pig. Endocrinology. 1957;60:607–618. doi: 10.1210/endo-60-5-607. [DOI] [PubMed] [Google Scholar]
- Krieg M. Characterization of the androgen receptor in the skeletal muscle of the rat. Steroids. 1976;28:261–274. doi: 10.1016/0039-128x(76)90114-8. [DOI] [PubMed] [Google Scholar]
- Kvorning T, Andersen M, Brixen K, Madsen K. Suppression of endogenous testosterone production attenuates the response to strength training: a randomized, placebo-controlled, and blinded intervention study. Am J Physiol. 2006;291:1325–1332. doi: 10.1152/ajpendo.00143.2006. [DOI] [PubMed] [Google Scholar]
- Kvorning T, Andersen M, Brixen K, Schjerling P, Suetta C, Madsen K. Suppression of testosterone does not blunt mRNA expression of myoD, myogenin, IGF, myostatin or androgen receptor post strength training in humans. J Physiol. 2007;578:579–593. doi: 10.1113/jphysiol.2006.122671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke M, Coffey D.The male accessory tissues: structure, androgen, and physiology The Physiology of the Reproduction 1994Raven Press: New York; 1435–1488.In: Knobil E, Neill J (eds). [Google Scholar]
- Mackey AL, Kjaer M, Dandanell Jorgensen S, Mikkelsen KH, Holm L, Dossing S, et al. The influence of anti-inflammatory medication on exercise-induced myogenic precursor cell responses in humans. J Appl Physiol. 2007;103:425–431. doi: 10.1152/japplphysiol.00157.2007. [DOI] [PubMed] [Google Scholar]
- Mauras N, Hayes V, Welch S, Rini A, Helgeson K, Dokler M, et al. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab. 1998;83:1886–1892. doi: 10.1210/jcem.83.6.4892. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971;170:421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Petrella J, Kim J, Cross J, Kosek D, Bamman M. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab. 2006;291:937–946. doi: 10.1152/ajpendo.00190.2006. [DOI] [PubMed] [Google Scholar]
- Powers ML, Florini JR. A direct effect of testosterone on muscle cells in tissue culture. Endocrinology. 1975;97:1043–1047. doi: 10.1210/endo-97-4-1043. [DOI] [PubMed] [Google Scholar]
- Ruizeveld de Winter JA, Trapman J, Vermey M, Mulder E, Zegers ND, van der Kwast TH. Androgen receptor expression in human tissues: an immunohistochemical study. J Histochem Cytochem. 1991;39:927–936. doi: 10.1177/39.7.1865110. [DOI] [PubMed] [Google Scholar]
- Shackleton CH, Phillips A, Chang T, Li Y. Confirming testosterone administration by isotope ratio mass spectrometric analysis of urinary androstanediols. Steroids. 1997;62:379–387. doi: 10.1016/s0039-128x(96)00253-x. [DOI] [PubMed] [Google Scholar]
- Singh R, Artaza J, Taylor W, Gonzalez-Cadavid N, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–5088. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, et al. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol. 2002;283:154–164. doi: 10.1152/ajpendo.00502.2001. [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab. 2006;91:3024–3033. doi: 10.1210/jc.2006-0357. [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Roth SM, Lee MI, Bhasin S. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol. 2003;285:197–205. doi: 10.1152/ajpendo.00370.2002. [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab. 2004;89:5245–5255. doi: 10.1210/jc.2004-0084. [DOI] [PubMed] [Google Scholar]
- Sottas PE, Saudan C, Schweizer C, Baume N, Mangin P, Saugy M. From population- to subject-based limits of T/E ratio to detect testosterone abuse in elite sports. Forensic Sci Int. 2008;174:166–172. doi: 10.1016/j.forsciint.2007.04.001. [DOI] [PubMed] [Google Scholar]
- WADA Technical document: reporting and evaluation guidance for testosterone, epitestosterone, T/E ratio and other endogenous steroids 2004. Available at
- Wilson JD. Androgen abuse by athletes. Endocr Rev. 1988;9:181–199. doi: 10.1210/edrv-9-2-181. [DOI] [PubMed] [Google Scholar]
- Yesalis CE.Incidence of anabolic steroid use: a discussion of methodological issues Anabolic Steroids in Sport and Exercise 1993Human kinetics: champaign, IL, USA; 49–69.(ed). [Google Scholar]