Abstract
We report the development of magneto-optic technology for the rapid quantitative diagnosis of malaria that may also be realizable in a noninvasive format. Hemozoin, the waste product of malarial parasitic action on hemoglobin, is produced in a form that under the action of an applied magnetic field gives rise to an induced optical dichroism characteristic of the hemozoin concentration. Here we show that precise measurement of this induced dichroism may be used to determine the level of malarial infection because this correlates, albeit in a complex manner throughout the infection cycle, with the concentration of hemozoin in the blood and tissues of infected patients. Under conservative assumptions for the production of hemozoin as a function of parasitemia, initial results indicate that the technique can match or exceed other current diagnostic techniques. The validity of the approach is confirmed by a small preliminary clinical trial on 13 patients, and measurements on live parasitized cells obtained from in vitro culture verify the possibility of producing in vivo diagnostic instrumentation.
INTRODUCTION
Malaria remains a major health problem in many parts of the world (1). In regions where it is endemic, effective treatment and eradication are often compromised by lack of access to rapid, accurate, and affordable diagnosis because, unfortunately, the best diagnostic tools currently available require a laboratory environment. Even in Europe, the number of cases and fatalities increases yearly, reflecting the increasing preference of Europeans to holiday in malarial-prevalent areas coupled with a reluctance to take antimalarial prophylaxes (2). Native Europeans have no acquired immunity so that without early diagnosis, infection can, and often does, have rapidly fatal consequences (16 United Kingdom deaths in 2003). This situation is, moreover, only likely to worsen as global warming is now predicted (3) to facilitate the spread of malaria to areas previously free of the disease including southern Europe.
Examination by high-power microscopy of typically 200 fields of Giemsa-stained thick blood smears is still generally regarded as the so called “gold standard” for malarial diagnosis (4). Dominant for more than a century this technique can in principle attain a sensitivity of better than 10 parasite-infected cells per microliter of blood. It is, however, time consuming and in reality subject to significant variability in its application, particularly in respect to the number of fields examined and the methodology employed to determine parasitemia from parasite counts within the fields examined (5). The technique is also dependent on the skill base of highly trained microscopists, and consequently, the highest sensitivities are rarely obtained outside specialist laboratories.
Recognition of this and of the need for more rapid diagnosis has, over the last decade or so, driven the study and development of several alternative techniques. These include fluorescent microscopy, laser desorption mass spectrometry (6,7), and techniques involving DNA/RNA amplification to detect and identify nucleic acid sequences that are currently acknowledged as the most sensitive and species specific (8). In general, however, this emerging generation of diagnostic procedures remains time consuming and again too costly and complex for dissemination beyond specialist laboratories. For field application there are now available from a variety of manufacturers rapid detection tests (RDTs) (4,9) in the form of lateral flow assays. These employ immunochromatographic methods to detect malarial antigens such as the histidine-rich protein II, aldolase (pan malarial antigen), or parasite-specific lactate dehydrogenase, which are present in peripheral blood during infection. These tests generate results within 15 min and require only minimal operator training. They are, however, relatively expensive compared to microscopy, nonquantitative, and have a limited usefulness in detecting low-level parasitemia (<100 parasites/μl). Despite the above developments, there remains a demonstrably pressing need for new diagnostic principles affording rapid yet simpler quantitative instrumentation suitable for field use or at first point symptom presentation. It would moreover be of immense benefit if new techniques could be rendered in a noninvasive form.
DIAGNOSTIC METHODOLOGY
During infection, malarial parasites invade erythrocytes and digest the protein (globin) part of the hemoglobin molecule. The heme component, which is toxic to the parasite, is converted into insoluble hemozoin in the form of rodlike crystals; the transformation of low-spin (Fe2+) diamagnetic oxyhemoglobin into high-spin (Fe3+) paramagnetic hemozoin produces a change in magnetic state and susceptibility, which has previously been used to concentrate parasitized cells to improve detection (10). Initially deposited in vacuoles within the erythrocytes, the hemozoin is subsequently released into suspension in the plasma on rupture of the parasitized erythrocytes, from where it is ultimately scavenged by leukocytes (11,12). Detection of the presence of hemozoin in blood or tissue is therefore positively indicative of malarial infection, and its concentration might be expected to correlate in some as yet unknown manner with the level of parasitemia.
Scanning electron micrographs show that the malarial parasite produces hemozoin crystals in a distinct rectangular form within a narrow size spectrum (13). As a result of their shape, these crystals are found to exhibit a high level of both magnetic anisotropy and optical dichroism, and it is these properties that are identified for exploitation as the basis of a new diagnostic technique. When suspended in a fluid, such as blood, the long axes of the hemozoin crystals are randomly orientated throughout three dimensions, and so the suspension expresses no preferred direction of optical absorption on interrogation using linearly polarized radiation. However, on application of a magnetic field, the paramagnetic crystals will become weak bar magnets experiencing a torque seeking to orient them along the applied field direction. This is opposed by the thermal energy of their environment, which constantly acts to randomize the assembly. At any nonzero field value, the total component of the crystals' long axes resolved along the field direction will be greater than that along the orthogonal to the field direction when integrated over all crystals in the dispersion. Optically this manifests as an induced dichroism, and, therefore, under the action of an applied magnetic field, any dispersion of hemozoin crystals behaves increasingly like a weak dichroic polarizer similar, when all crystals are fully orientated with the field, to Polaroid. Magneto-optically the phenomenon is directly analogous to the Cotton-Mouton effect. Interrogation of dispersions of magnetically aligned hemozoin crystals with optical beams in which the polarization is modulated between linear states aligned along and orthogonal to the applied field direction therefore produces an optical modulation signal that, when expressed as a fraction of the sample transmittance (or absorption), is directly proportional to the crystal concentration, affording, in principle, a simple and precise optical technique for the diagnosis of parasitemia.
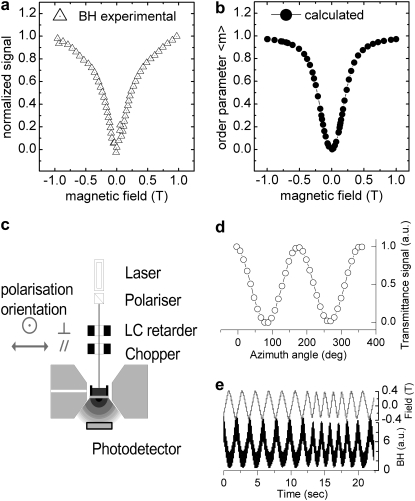
The validity of this mechanism and its potential as a technique for the detection of malaria and quantification of the level of parasitemia are initially explored by studying the optical response of suspensions of β-hematin crystals in phosphate-buffered saline (PBS) to the imposition of an applied magnetic field. β-Hematin is a readily synthesized analog of hemozoin that all references cite as its complete equivalent (14–16). In our hands, the method of Slater et al. (14) yields β-hematin crystals some 0.78 μm in length with a cross section ∼0.22 μm square. This is comparable with hemozoin crystals sourced from cultured parasitic cells, although the standard distribution of these dimensions is currently somewhat larger for the synthesized crystals. Fig. 1 a shows the increasing dichroic signal imposed on a beam of 660-nm optical radiation during transmission through a 20 μg/ml dispersion of β-hematin crystals in PBS when an applied magnetic field is cycled between values of ±1 T.
FIGURE 1.
(a) Difference in transmission (normalized to value at 1 T) for orthogonal polarized beams as a result of magnetically induced dichroism in a β-hematin (BH) suspension (20 μg/ml). (b) Magnetic orientation of crystals suspended in fluid modeled following formalism described in text. (c) Experimental configuration. (d) Magnetically aligned 20 μg/ml concentration of BH set in 10% w/v gelatin gel behaving as a dichroic polarizer. (e) Dynamic response of dispersion of BH to alternating magnetic field.
Equivalent concentrations of purified hemozoin extracted from parasitized cells give an identical response to within 10%. It is clear that at the maximum field values the mechanism is approaching saturation as the crystals come into near full alignment with the field. Moreover, following a formalism analogous to that employed by O'Konski et al. (17) to describe the orientation of molecules in a strong electric field, the form of the behavior observed in Fig. 1 a may be modeled in terms of an order parameter of the crystals that has a value of 0 when crystals are randomly dispersed and 1 when fully aligned along the field as shown in Fig. 1 b. The experimental and theoretical plots shown in Fig. 1, a and b, respectively, are obtained in the configuration shown in Fig. 1 c with the magnetic field applied in the plane of the liquid surface and the optical polarization modulated electro-optically at frequencies of either 10 Hz or 44 kHz. If, however, the field is applied perpendicular to the liquid surface, then although the absorption for both polarization states decreases equally with field until full alignment is obtained, no response under polarization modulation is observed because the random in-plane distribution of crystals is maintained as they orientate with the field. Further evidence confirming the nature of the conjectured mechanism underlying the observed changes in dichroism is provided by suspending β-hematin crystals at the same 20 μg/ml concentration in gelatin (10%, w/v) rather than PBS and then allowing it to set from its liquid state at ∼60°C. This produces samples in which the orientation state of the crystals is fixed. Samples setting in an applied in-plane magnetic field subsequently behave as weak polarizers as shown in Fig. 1 d, where the transmission as a function of azimuth angle is plotted as such a sample is rotated in a beam of linearly polarized radiation. Conversely, those setting in the absence of a field retain the thermally randomized state and, as expected, exhibit no such behavior. Finally, in addition to these measurements on constrained crystals, initial studies into the dynamics of the orienting mechanism are conducted by probing the transmission of a 20 μg/ml suspension of β-hematin with an optical beam in a single linear state of polarization while the frequency of the applied magnetic field is varied. The constancy of the dichroic signal amplitude with magnetic field frequency in Fig. 1 e shows that the rotation of the crystal rods easily follows the magnetic driving and thermal restoring forces up to frequencies of 10 Hz, the highest currently achievable with our electromagnets. At these frequencies, viscous damping is of little importance.
RESULTS
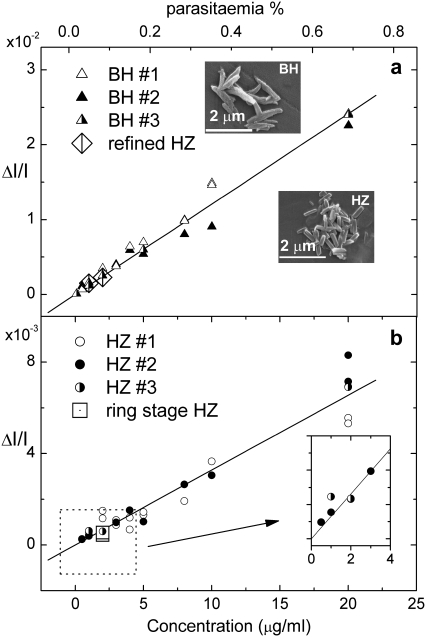
Once the nature of the diagnostic principle has been established, its potential is first evaluated by mimicking the blood hemozoin content arising from different levels of malarial infection and measuring its fractional change in transmittance at 660 nm on imposition of a 1-T in-plane magnetic field. Fig. 2 a displays the results for whole fresh blood doped with β-hematin crystals dispersed by sonication, whereas the results in Fig. 2 b are obtained by replacing the β-hematin with hemozoin in the form of mature trophozoite stage parasitized red blood cells (PRBCs) grown in culture and lysed (ruptured) by freezing and thawing before diluting with whole fresh blood. In both these batches of artificially created malarial samples, the uncertainty in the hemozoin (β-hematin) concentration is dependent on the concentration but ranges between 2.9% at the higher concentrations to ∼10% at the lowest. The linearity of both plots is, however, striking and continues (not shown) out to beyond at least 100 μg/ml. Note also how the data point obtained with cells in which the infection is at the early ring stage of hemozoin formation, when the crystals are believed to be much smaller, fits closely to the linear trend. In this ring stage sample, the conversion level of hemoglobin heme to hemozoin is determined <0.5% per PRBC. This is consistent with the low levels of hemozoin expected in the young parasite stages and is of the order of 100 times lower than the conversion rate determined for later trophozoite stage culture material.
FIGURE 2.
Fractional change in transmittance ΔI/I associated with magnetically induced dichroism. (a) Differing concentrations of β-hematin dispersed in whole fresh blood, and (b) differing concentrations of hemozoin in the form of parasitized red blood cells (PRBCs) grown in culture and lysed by freezing before dilution with whole fresh blood. (Insets) Electron micrographs of synthesized β-hematin (BH) and hemozoin (HZ) extracted from cultured cells. Legends 1, 2, and 3 refer to samples mixed and measured on different occasions. Correlation between the hemozoin concentration and parasitemia scales on both the upper and lower graphs is made as described in the text.
The difference in the gradients of the plots is attributed a consequence of at least two factors. First, hemozoin crystals in situ exhibit a tendency to clump (18), which reduces the dichroism when compared with an equivalent number of individual crystals, such as is the case with β-hematin. Second, cultured cells are supplied frozen to ensure all cells are at the same point in their infection cycle and to facilitate safe handling. Freezing, however, appears to only partially release the hemozoin so that cellular debris continues to adhere to the crystals, altering the dynamic forces acting on them and thereby constraining the rotation achieved in a given applied field. Adherence of diamagnetic cellular material to the crystals would, for example, result in a small torque opposing that seeking to orient the crystals with the field. Alternatively, adhering cellular material might simply be providing a larger interaction cross-section for the thermal restoring mechanism. Plots obtained in the same way as Fig. 1 a for blood doped in this manner have exactly the same form but with reduced dichroism. Similarly, the rate of response of the dichroic signal to changes in the applied field is also observed to be reduced. In developed instrumentation, a detergent will be used to completely release the hemozoin to restore the dichroic signal strength to that exhibited by an equivalent β-hematin concentration. The effectiveness of this procedure is tested by extracting the hemozoin from two of the samples of PRBCs used in producing Fig. 2 b. This is detergent-washed before being reintroduced at the same concentration into whole fresh blood. When the fractional intensity change for these samples is then plotted (R) with the free β-hematin data in Fig. 2 a, it is in very close agreement.
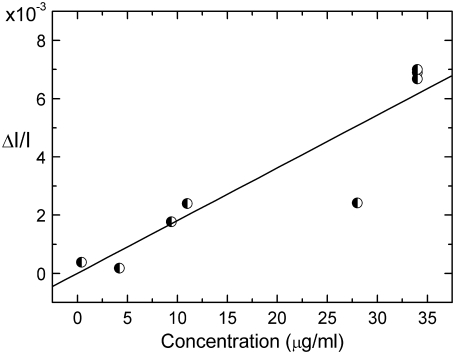
Fig. 3 shows the fractional change in absorption arising from magnetically induced dichroism when the experiments whose results are depicted in Fig. 2 are repeated using fresh blood containing varying concentrations of live parasitized cells. Samples for this experiment were harvested randomly from a nonsynchronized parasitized culture in which the concentration of parasitized cells varied throughout the culture feeding cycle. Although in these samples all hemozoin remains secreted in the cell vacuoles, the results obtained are comparable to those of Fig. 2 b for lysed blood. This demonstrates that hemozoin remains free to rotate within the vacuoles and still able to orient either partially or fully with the applied magnetic field. Close comparison of Fig. 3 with Fig. 2 b reveals however a further slight suppression of the gradient. This is because, in each sample taken, the nonsynchronized nature of the culture produces parasitized cells containing hemozoin in all its developmental states. Given the finite size of the vacuoles, it is unlikely that the freedom of the hemozoin crystals to rotate within them and hence contribute fully to the detected signal will remain constant as their size increases through (early) ring, trophozoite, and schizont stages. The magnitude of the signal arising from schizont stages will be particularly susceptible to suppression disproportionate to their number given the known tendency of hemozoin crystals associated with this state to adhere into large clumps. Again, this explanation is confirmed by subsequently lysing samples when the signal returns to correspondence with that obtained for an equivalent concentration in Fig. 2 b. In all the above experiments, the concentration of hemozoin in the samples was determined using spectroscopic absorption measurements.
FIGURE 3.
Fractional change in transmittance ΔI/I associated with magnetically induced dichroism arising from rotation of hemozoin crystals within the cell vacuoles of live parasitized cells grown and sampled from nonsynchronous culture.
Analytical performance of magneto-optical test device
A portable demonstrator instrument operating on the diagnostic principles described was developed and evaluated in a small preclinical trial. After informed consent had been obtained, blood samples from study cases with either confirmed malaria, undifferentiated fever symptoms, rheumatic-associated disease, or hemoglobinopathies (see Table 1) were tested for Plasmodium infection with RDT and magneto-optical test (MOT). The samples studied had been transported or stored frozen and were further lysed and diluted by the addition of triton detergent before being offered blind to the instrument operator. Table 1 reveals that there was an excellent correlation among MOT testing, RDT results, and clinical confirmation. The ease with which the system detected a Plasmodium ovale infection, known for its low parasitemia, is particularly encouraging.
TABLE 1.
Results of evaluating a small set of clinical samples of individuals with fever or no fever but from a disease-endemic country with health complaints
| Patient characteristics | Result rapid diagnostic test for malaria | Final clinical diagnosis based on extensive diagnostic procedures | MOT diagnosis | MOT fractional change ΔI/I |
|---|---|---|---|---|
| Nigerian child with fever | Positive | P. falciparum infection | Positive | 8.2E-5 |
| Nigerian child with fever | Positive | P. falciparum infection | Positive | 4.7E-5 |
| Nigerian child with fever | Positive | P. falciparum infection | Positive | 7.8E-5 |
| Nigerian child with fever | Positive | P. falciparum infection | Positive | 9.5E-5 |
| Nigerian child with fever | Positive | P. falciparum infection | Positive | 5.8E-5 |
| Dutch returned traveler | Positive | P. ovale infection | Positive | 4.4E-5 |
| Dutch returned traveler | Positive | P. falciparum infection | Positive | 5.9E-3 |
| Tanzanian adult; asymptomatic for malaria | Negative | Sickle cell anemia | Negative | — |
| Tanzanian adult; asymptomatic for malaria | Negative | β-thalassemia | Negative | — |
| Tanzanian adult; asymptomatic for malaria | Negative | Rheumatic-associated disease | Negative | — |
| Dutch returned traveler with fever | Negative | Undifferentiated fever | Negative | — |
| Dutch returned traveler with fever | Inconclusive | Undifferentiated fever | Negative | — |
| Dutch returned traveler with fever | Negative | Visceral leishmaniasis | Negative | — |
DISCUSSION
The evidence presented, and the preclinical trial data in particular, clearly validates this new diagnostic principle for working with drawn blood. However, to fully evaluate its potential worth and critically compare its ultimate sensitivity against existing techniques of this type requires making assumptions regarding the relation between the accessible hemozoin load in the bloodstream and parasitemia. Unfortunately, reliable quantitative data that might facilitate this are scarce, and even less is known about the elimination of hemozoin from the body (19). To create the comparative upper and lower axes for Fig. 2, it was assumed that whole blood contains ∼5 × 109 RBCs/ml and that in mature parasitized cells conversion of 50% of the hemoglobin yields ∼0.6 pg hemozoin per cell (20,21). On this basis, detecting 100 PRBCs/μl (0.002% parasitemia), for example, requires measurement of hemozoin concentrations ∼0.06 μg/ml. Although the lowest created-to-calibration concentration of hemozoin measured to date is 0.01 μg/ml (in PBS), as shown by the linear separation of the lower data points in Fig. 2, a and b, the resolution of the instrumentation, even in prototype form, is actually somewhat better than this.
Moreover, in this initial work neither the sample volume nor the optical path length has as yet been optimized to maximize the detectable fractional intensity change. The measured parameter (ΔI/I) is of course a function of the optical path length, and ultimately the sensitivity achievable will for the most part be determined by the degree to which the orthogonal polarization states used to interrogate the induced dichroism are depolarized by scattering from cellular structures and other material during their passage through the blood sample before they interact with hemozoin crystals. Loss of polarization after this point is unimportant because only the resulting intensity modulation is detected. In this context, it is noted that hemozoin is itself known to depolarize light, and the additional depolarization produced by its presence in blood has been previously explored as a means of diagnosing malaria (22). In principle there is no reason why the technique cannot readily be refined to permit measurement of fractional intensity changes with more precision than the currently achieved value of ∼5 × 10−6. Values lower than this are regularly attained in optical and magneto-optical measurements (23–25). A measurement precision of 1 × 10−6 would, for example, render possible the rapid automatic detection of parasitemia levels approaching 0.0002% (10 PRBCs/μl) by low-skilled operators even in the case of Plasmodium falciparum when only the ring stage exhibits a significant presence in the circulatory system and for which the above assumption of hemoglobin conversion may be optimistic by up to a factor of 50.
Other working methodologies may then offer yet further improvements or simplification. For example, rather than continue with the ratiometric methodology in which evaluation of the fractional intensity change for correlation with malarial parasitemia requires two independent measurements, the same level of detection sensitivity might be more readily obtained by employing a dual-beam differential system in which only one of two identically sourced samples is subject to an applied magnetic field.
Although the simplicity and rapidity of diagnosis offered by instrumentation working as described above represent a significant improvement on existing methods, the real importance of this technology lies in its potential to function in an in vivo or noninvasive mode precluding the need to draw and handle blood. During infection with P. falciparum, a certain population of the mature PRBCs sequester in the microcirculation within organs and subcutaneous tissues (26,27). A cumulative measurement of the hemozoin present in both circulating and sequestered PRBCs together with that phagocytosed in peripheral and intradermal leukocytes and liberated in the blood vessels on schizont rupture may give a better indication of the patient's actual parasite load and thus provide a better prognostic indicator (28,29). Hemozoin has been shown free to respond to the detection process whether liberated in the bloodstream or confined within cell vacuoles.
Similar studies seeking to confirm the freedom of hemozoin ingested by live leukocytes and its relation to the observed fractional intensity change in an applied magnetic field have thus far proved positive but inconclusive because of the difficulty of removing the excess hemozoin not taken up by the leukocytes. However, microscopic observation confirms, as indicated elsewhere (30,31), that hemozoin is indeed free to respond in live leukocytes. Responsive to the total hemozoin load, a noninvasive measurement, across say the fingertip or earlobe in the manner of a pulse oximetry device, therefore appears to promise potentially more sensitivity than the blood-sampling instrument. Unfortunately, scattering, which is much more pronounced in skin and tissue than in blood and lysed blood in particular, rapidly destroys the incident state(s) of polarization.
Initially it might therefore appear that the polarization modulation technique described previously will be unsuccessful in a noninvasive mode. However, Pircher et al. (32) indicate that although the stratum corneum randomizes the polarization state of light backscattered from within it, contrary to previous belief, it has little impact on light progressing to the lower dermal levels so that some considerable degree of incident polarization is retained to depths of at least 800 μm and beyond. If this is the case, then sensitive noninvasive access to all hemozoin within most of the capillary-rich dermis becomes possible because, as pointed out above, loss of polarization after interaction with a magnetically orientated crystal is unimportant because only the resulting intensity modulation is detected. Moreover, if nail were to prove similarly polarization transparent, then interrogation of the nail bed may be especially sensitive, particularly if done in the configuration in which the magnetic field is applied co-linear to the optical beam when the lightwave-hemozoin interaction functions even with unpolarized light. Other changes might be necessary or desirable to optimize performance in a noninvasive mode. First, it may be essential to operate at the isobestic point (λ ≈ 800 nm rather than 660 nm) to preclude or reduce interference from the pulse. Second, after a full safety evaluation, magnetic field modulation may be a preferable route to synchronous signal modulation/detection. Finally, it is obvious that the relation between ΔI/I and hemozoin concentration for noninvasive measurements will be significantly different from those given in Figs. 2 and 3 and will be determinable only through clinical trials.
Instrumentation operating in invasive (blood sampling) mode is now under construction and scheduled for field trials in Africa in late 2008. Research continues on the possibility of implementing the diagnostic technique in noninvasive format.
Acknowledgments
We thank Robert Pinches at The Weatherall Institute of Molecular Medicine, John Radcliffe Hospital, Oxford, United Kingdom, for the provision of in vitro P. falciparum cultures. We also thank the study participants for their willingness to participate in this study.
This work has been performed under “Novel Magneto-Optical Biosensors for Malaria Diagnosis” of the European Commission Framework 6 Program (http://www.mottest.org).
Authors Dave M. Newman and John Heptinstall conceived this technique. All other authors made broadly equal contributions to the program.
Editor: Gerard Marriott.
References
- 1.Greenwood, B. M., K. Bojang, C. J. M. Whitty, and G. A. T. Targett. 2005. Malaria. Lancet. 365:1487–1498. [DOI] [PubMed] [Google Scholar]
- 2.Toovey, S., and A. Jamieson. 2003. Rolling back malaria: how well is Europe doing? Travel Med. Infect. Dis. 1:167–175. [DOI] [PubMed] [Google Scholar]
- 3.McMichael, A. J., R. E. Woodruff, and S. Hales. 2006. Climate change and human health: present and future risks. Lancet. 367:859–869. [DOI] [PubMed] [Google Scholar]
- 4.Moody, A. 2002. Rapid diagnostic tests for malaria parasites. Clin. Microbiol. Rev. 15:66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makler, M. T., C. J. Palmer, and A. L. Ager. 1998. A review of practical techniques for the diagnosis of malaria. Ann. Trop. Med. Parasitol. 92:419–433. [DOI] [PubMed] [Google Scholar]
- 6.Kawamoto, F. 1991. Rapid diagnosis of malaria by fluorescence microscopy with light microscope and interference filter. Lancet. 337:200–202. [DOI] [PubMed] [Google Scholar]
- 7.Scholl, P. F., D. Kongkasuriyachai, P. A. Demirev, A. B. Feldman, J. S. Lin, D. J. Sullivan, Jr., and N. Kumar. 2004. Rapid detection of malaria infection in vivo by laser desorption mass spectrometry. Am. J. Trop. Med. Hyg. 71:546–551. [PubMed] [Google Scholar]
- 8.Mens, P. F., G. J. Schoone, P. A. Kager, and H. D. F. H. Schallig. 2006. Detection and identification of human Plasmodium species with real-time quantitative nucleic acid sequence-based amplification. Malar. J. 5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hänscheid, T. 1999. Diagnosis of malaria: a review of alternatives to conventional microscopy. Clin. Lab. Haematol. 21:235–245. [DOI] [PubMed] [Google Scholar]
- 10.Nalbandian, R. M., D. W. Sammons, M. Manley, L. Xie, C. R. Sterling, N. B. Egen, and B. A. Gingras. 1995. A molecular-based magnet test for malaria. Am. J. Clin. Pathol. 103:57–64. [DOI] [PubMed] [Google Scholar]
- 11.Bohle, D. S., P. Debrunner, P. A. Jordan, S. K. Madsen, and C. E. Schulz. 1998. Aggregated heme detoxification byproducts in malarial trophozoites: β-hematin and malaria pigment have a single S = 5/2 iron environment in the bulk phase as determined by EPR and magnetic Mössbauer spectroscopy. J. Am. Chem. Soc. 120:8255–8256. [Google Scholar]
- 12.Metzger, W. G., B. G. Mordmüller, and P. G. Kremsner. 1995. Malaria pigment in leucocytes. Trans. R. Soc. Trop. Med. Hyg. 89:637–638. [DOI] [PubMed] [Google Scholar]
- 13.Noland, G. S., N. Briones, and D. J. Sullivan, Jr. 2003. The shape and size of hemozoin crystals distinguishes diverse Plasmodium species. Mol. Biochem. Parasitol. 130:91–99. [DOI] [PubMed] [Google Scholar]
- 14.Slater, A. F. G., W. J. Swiggard, B. R. Orton, W. D. Flitter, D. E. Goldberg, A. Cerami, and G. B. Henderson. 1991. An iron-carboxylate bond links the heme units of malaria pigment. Proc. Natl. Acad. Sci. USA. 88:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohle, D. S., R. E. Dinnebier, S. K. Madsen, and P. W. Stephens. 1997. Characterization of the products of the heme detoxification pathway in malarial late trophozoites by x-ray diffraction. J. Biol. Chem. 272:713–716. [DOI] [PubMed] [Google Scholar]
- 16.Pagola, S., P. W. Stephens, D. S. Bohle, A. D. Kosar, and S. K. Madsen. 2000. The structure of malaria pigment β-haematin. Nature. 404:307–310. [DOI] [PubMed] [Google Scholar]
- 17.O'Konski, C. T., K. Yoshioka, and W. H. Orttung. 1959. Electric properties of macromolecules. IV. Determination of electric and optical parameters from saturation of electric birefringence in solutions. J. Phys. Chem. 63:1558–1565. [Google Scholar]
- 18.Goldberg, D. E., A. F. G. Slater, A. Cerami, and G. B. Henderson. 1990. Hemoglobin degradation in the malaria parasite Plasmodium falciparum: An ordered process in a unique organelle. Proc. Natl. Acad. Sci. USA. 87:2931–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanscheid, T., T. J. Egan, and M. P. Grobusch. 2007. Haemozoin:from melatonin pigment to drug target, diagnsotic tool, and immune modulator. Lancet Infect. Dis. 7:675–685. [DOI] [PubMed] [Google Scholar]
- 20.Orjih, A. U., and C. D. Fitch. 1993. Hemozoin production by Plasmodium falciparum: variation with strain and exposure to chloroquine. Biochim. Biophys. Acta. 1157:270–274. [DOI] [PubMed] [Google Scholar]
- 21.Egan, T. J., J. M. Combrinck, J. Egan, G. R. Hearne, H. M. Marques, S. Ntenteni, B. T. Sewell, P. J. Smith, D. Taylor, D. A. van Schalkwyk, and J. C. Walden. 2002. Fate of haem iron in the malaria parasite Plasmodium falciparum. Biochem. J. 365:343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wrona, J., T. Stobiecki, M. Czapkiewicz, R. Rak, T. Śle Zak, J. Korecki, and C. G. Kim. 2004. R-VSM and MOKE magnetometers for nanostructures. J. Magn. Magn. Mater. 272–276:294–295. [Google Scholar]
- 23.Mendelow, B. V., C. Lyons, P. Nhlangothi, M. Tana, M. Munster, E. Wypkema, L. Liebowitz, L. Marshall, S. Scott, and T. L. Coetzer. 1999. Automated malaria detection by depolarization of laser light. Br. J. Haematol. 104:499–503. [DOI] [PubMed] [Google Scholar]
- 24.Hollister, J. H., G. R. Apperson, L. L. Lewis, T. P. Emmons, T. G. Vold, and E. N. Fortson. 1981. Measurement of parity nonconservation in atomic bismuth. Phys. Rev. Lett. 46:643–646. [Google Scholar]
- 25.Drake, A. F. 1986. Polarisation modulation-the measurement of linear and circular dichroism. J. Phys. E Sci. Instrum. 19:170–181. [Google Scholar]
- 26.MacPherson, G. G., M. J. Warrell, N. J. White, S. Looareesuwan, and D. A. Warrell. 1985. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am. J. Pathol. 119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 27.Wilairatana, P., M. Riganti, P. Puchadapirom, B. Punpoowong, S. Vannaphan, R. Udomsangpetch, S. Krudsood, G. M. Brittenham, and S. Looareesuwan. 2000. Prognostic significance of skin and subcutaneous fat sequestration of parasites in severe falciparum malaria. Southeast Asian J. Trop. Med. Public Health. 31:203–212. [PubMed] [Google Scholar]
- 28.Dondorp, A. M., V. Desakorn, W. Pongtavornpinyo, D. Sahassananda, K. Silamut, K. Chotivanich, P. N. Newton, P. Pitisuttithum, A. M. Smithyman, N. J. White, and N. P. Day. 2005. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2:788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phu, N. H., N. Day, P. T. Diep, D. J. P. Ferguson, and N. J. White. 1995. Intraleucocytic malaria pigment and prognosis in severe malaria. Trans. R. Soc. Trop. Med. Hyg. 89:200–204. [DOI] [PubMed] [Google Scholar]
- 30.Tiffert, T., H. M. Staines, J. C. Ellory, and V. L. Lew. 2000. Functional state of the plasma membrane Ca2+ pump in Plasmodium falciparum-infected human red blood cells. J. Physiol. 525:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biagini, G. A., P. G. Bray, D. G. Spiller, M. R. H. White, and S. A. Ward. 2003. The digestive food vacuole of the malaria parasite is a dynamic intracellular Ca2+ store. J. Biol. Chem. 278:27910–27915. [DOI] [PubMed] [Google Scholar]
- 32.Pircher, M., E. Goetzinger, R. Leitgeb, and C. K. Hitzenberger. 2004. Three dimensional polarization sensitive OCT of human skin in vivo. Opt. Express. 12:3236–3244. [DOI] [PubMed] [Google Scholar]