Table 1.
Asymmetric Petasis Reaction Catalyzed by Chiral Diolsa
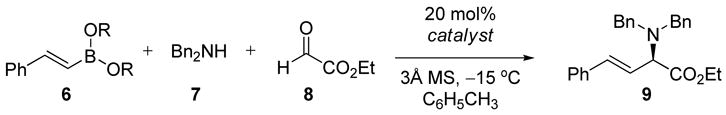 | |||||
|---|---|---|---|---|---|
| entry | boronate | R | catalyst | % yieldb | erc |
| 1 | 6a | H | - | 80 | - |
| 2 | 6b | i-Pr | - | <5 | - |
| 3 | 6b | i-Pr | 5a | 45 | 60:40 |
| 4 | 6b | i-Pr | 5b | 65 | 75:25 |
| 5 | 6b | i-Pr | 5c | 51 | 70:30 |
| 6 | 6b | i-Pr | 5d | 25 | 59:41 |
| 7 | 6b | i-Pr | 5e | 70 | 55:45 |
| 8 | 6b | i-Pr | 5f | 60 | 70:30 |
| 9 | 6b | i-Pr | 5g | 43 | 64:36 |
| 10 | 6b | i-Pr | 5h | 67 | 72:28 |
| 11 | 6b | i-Pr | 5i | 77 | 85:15 |
| 12 | 6b | i-Pr | 5j | 80 | 87:13 |
| 13 | 6c | CH3 | 5j | 90 | 90:10 |
| 14 | 6d | Et | 5j | 81 | 95.5:4.5 |
| 15 | 6e | n-Bu | 5j | 77 | 93:7 |
| 16 | 6a | H | 5j | 90 | 57:43 |
Reactions were run with 0.15 mmol boronate, 0.10 mmol dibenzylamine, 0.10 mmol glyoxylate, 0.020 mmol catalyst, and 3Å molecular sieves in toluene (0.1 M) for 24 h under Ar, followed by flash chromatography on silica gel.
Isolated yield.
Enantiomeric ratios determined by chiral HPLC analysis.
