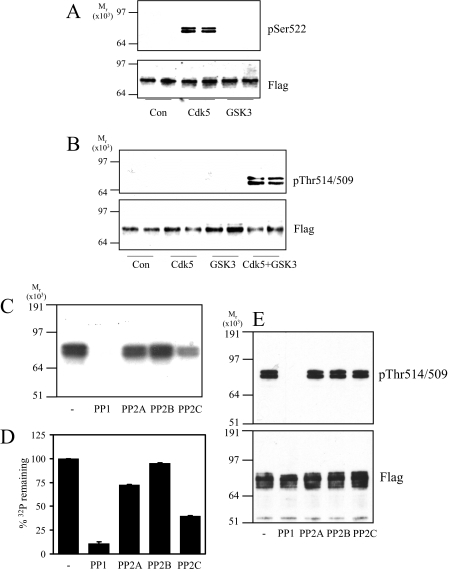
FIGURE 1.
Dephosphorylation of recombinant CRMP2 by phosphatases in vitro. A, GST-FLAG-CRMP2 was incubated alone (Con) or with Cdk5 or GSK3β in the presence of unlabeled ATP. Phosphorylation was detected using an antibody that specifically recognizes CRMP2 when phosphorylated at Ser-522 (upper panel) or an antibody that recognizes the FLAG tag (lower panel). B, GST-FLAG-CRMP2 was incubated alone (Con) or with Cdk5, GSK3β, or Cdk5 plus GSK3β in the presence of unlabeled ATP. Phosphorylation was detected using an antibody that specifically recognizes CRMP2 when phosphorylated at Thr-514/509 (upper panel) and a FLAG tag antibody (lower panel). C, GST-FLAG-CRMP2 was primed by Cdk5 in the presence of unlabeled ATP followed by incubation with GSK3β and [γ-32P]ATP. The [γ-32P]CRMP2 was then incubated with 50 milliunits of each of the phosphatases listed for 1 h. After SDS-PAGE, [γ-32P]CRMP2 was visualized by autoradiography. D, the amount of phosphate remaining in CRMP2 in C was measured by Cerenkov counting of the relevant gel pieces and is presented as a percentage of control (no phosphatase added) (n = 3). E, GST-FLAG-CRMP2 was phosphorylated by Cdk5 and GSK3β in the presence of unlabeled ATP, then incubated with 50 milliunits of the phosphatases listed for 1 h. The extent of dephosphorylation was assessed using antibodies to Thr(P)-514/509 (upper panel) and FLAG tag (lower panel) (n = 2).