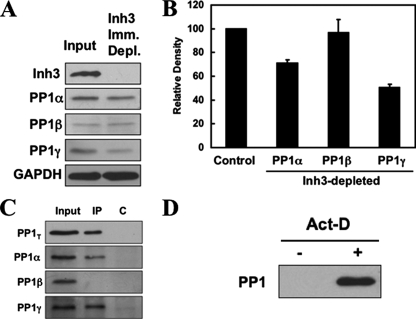
FIGURE 6.
Inh3 is associated with significant fractions of the PP1α and PP1γ1 isoforms in HL-60 cells. HL-60 cell lysates were immunoprecipitated with a polyclonal antibody against Inh3 using amounts of antibody that were predetermined to be sufficient to immunodeplete the lysates (see “Experimental Procedures”). A, the Inh3-immunodepleted supernatants were Western blotted with antibodies against Inh3 and with isoform-specific antibodies against PP1α, PP1β, or PP1γ1, as indicated. GAPDH was used as a loading control. B, the amounts of PP1α, PP1β, and PP1γ1 remaining in the Inh3-depleted supernatants relative to the input (Control) were determined by densitometric analysis (see “Experimental Procedures”) and shown as a bar diagram. The data are presented as mean ± S.D. (n = 3). C, the immunoprecipitates (IP) from the experiment shown in A were immunoblotted with a non-isoform-specific antibody against PP1 (shown as PP1T) or with isoform-specific antibodies against PP1α, PP1β, or PP1γ1. The lanes shown are the input, the immunoprecipitate (IP), and the immunoprecipitation performed with normal rabbit IgG (lane C). D, pull-down assays of untreated and act-D-treated HL-60 lysates for free PP1. HL-60 cells were treated with 4 μm act-D for 5 h. Cell lysates were pulled down using TALON metal affinity beads that were presaturated with His-Inh3. The bound proteins were extracted with loading buffer and subjected to SDS-PAGE and immunoblotted with antibody against PP1 (non-isoform-specific).