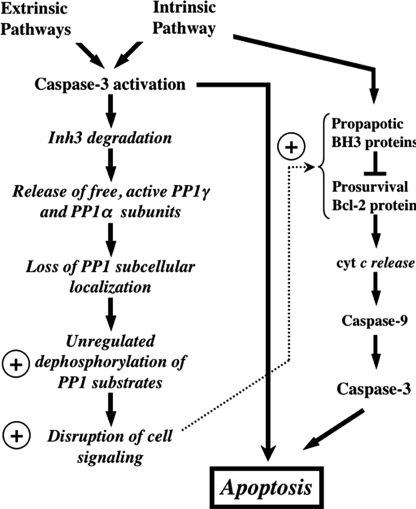
FIGURE 8.
Overview of the potential impact of caspase-3 degradation of Inh3 on cell functions. This diagram shows the potential impact of caspase-3 on Inh3 degradation within a simplified scheme of the apoptotic pathways. Since caspase-3 is a primary executioner caspase, Inh3 degradation is expected to accompany caspase-3-activated apoptotic signaling pathways whether these are generated through the intrinsic or extrinsic pathways. Inh3 is a potent PP1 inhibitor and controls a significant fraction of the cellular pool of PP1γ1 and PP1α. In addition, it also controls the subcellular localization of PP1γ1 to the nucleolus. Degradation of Inh3 leads to release of active PP1 catalytic subunits, which are no longer restrained to their normal subcellular compartments. As a consequence, unregulated dephosphorylation of PP1 substrates by the active PP1 catalytic subunits takes place, leading to disruption of cellular processes. The circled plus sign is used here to indicate a proapoptotic effect. Among the PP1 substrates are a subset of those that are involved in apoptotic signaling pathways. Their unregulated dephosphorylation might also affect apoptotic signaling. On the right side of the diagram is a simplified outline of the intrinsic pathway to indicate the interactions of the Bcl-2 and BH3 family of proteins, of which several, including Bcl-2 and BAD, have been reported to be regulated by protein phosphatase-1 activity. Unregulated dephosphorylation of these proteins could produce a proapoptotic signaling effect. This would provide a potential feedback mechanism that is shown by the dotted arrow.