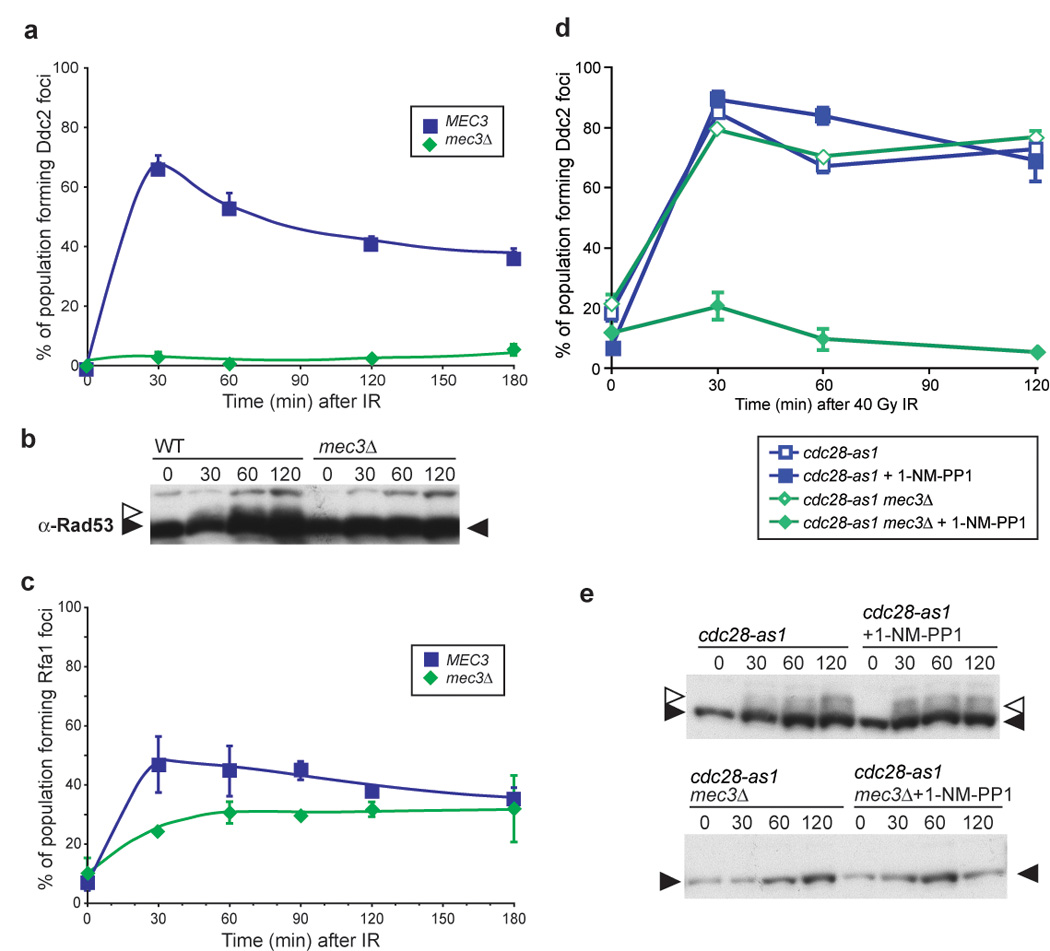
Figure 5. Independent recruitment of checkpoint machinery 9-1-1 and Ddc2-Mec1 requiresCdc28 activity.
(a) Ddc2 focus formation in mec3Δ G1 cells. mec3Δ cells were arrested in 2 µg/ml α-factor for 90 minutes and the cultures subsequently exposed to IR, and images were acquired at the stated timepoints. Ddc2 focus formation does not occur in mec3Δ cells (W5358-9A) arrested in G1.
(b) Rad53 phosphorylation in WT and mec3Δ G1 cells. Rad53 is phosphorylated in WT G1 cells after exposure to 40 Gy IR, while mec3Δ cells do not.
(c) Rfa1 focus formation in mec3Δ G1 cells. Deletion of MEC3 does not prevent Rfa1 focus formation during G1 (W5793-10B).
(d) Ddc2 focus formation in S/G2 mec3Δ cells with and without Cdc28 kinase activity. Ddc2 forms foci in budded cells, regardless of the presence of an active DNA damage clamp. When Cdc28 kinase activity is abrogated by the addition of the inhibitor 1-NM-PP1 to a strain containing the cdc28-as1 allele (W7832-2A), no Ddc2 focus formation is observed after 40 Gy IR, indicating that Cdc28 kinase activity is required for recruitment of Ddc2-Mec1 to the sites of DNA damage when Mec3 is absent (W7832-1A).
(e) Rad53 phosphorylation in WT and mec3Δ cells. Inhibition of cdc28-as1 cells with 1-NM-PP1 does not alter Rad53 phosphorylation in WT or mec3Δ cells.
