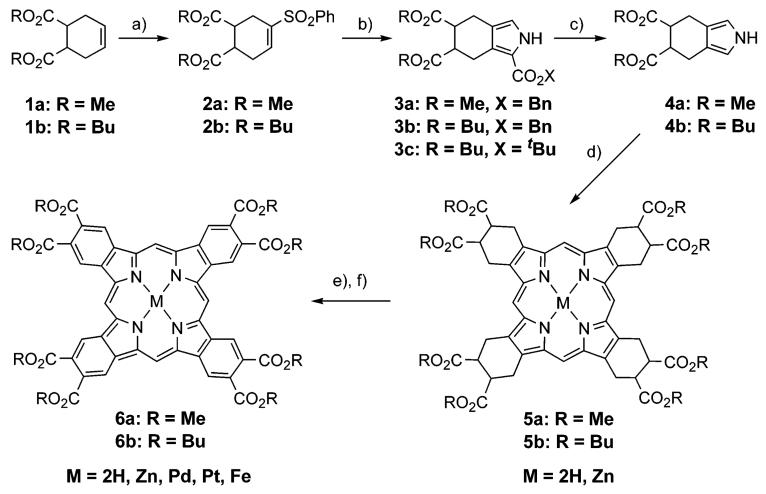
SCHEME 1a. a Reagents and conditions.
(a) (i) PhSCl, CH2Cl2, rt; (ii) MCPBA, CH2Cl2; (iii) DBU (80-85% for 3 steps); (b) CNCH2CO2X, tBuOK, THF, 0 °C (60-95%); (c) for R = Bn: (i) H2, Pearlman catalyst, THF-MeOH-Et3N, rt; (ii) (CH2OH)2, reflux, 30 min (82-90% for 2 steps); for R = tBu: TFA-CH2Cl2, Ar, rt, 30 min (30-40%); (d) (i) (CH2O)n, PhH, TsOH·H2O, Ar, reflux, 6-8 h, then air, (rt), overnight (30-45%); (ii) for M = Zn: Zn(OAc)2·2H2O, THF, reflux, 15 min (95-97%); (e) R = Bu: DDQ, toluene, reflux (95-97%); R = Me: DDQ, dioxane, reflux (20-96%); (f) PdCl2 or PtCl2, PhCN, reflux, Pd: 5-10 min, Pt: 7-8 h (84-90%), or Fe/FeCl2·4H2O, CH3CH2CO2H, Ar, reflux 1 h (74%).