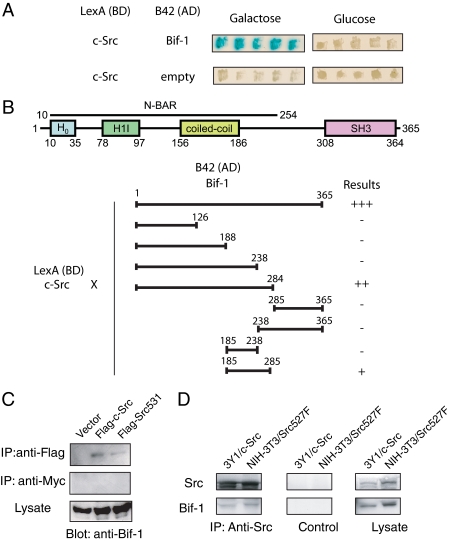
FIGURE 2.
Bif-1 interacts with Src in both yeast and mammalian cells. A, LexA-c-Src and B42-Bif-1 fusion proteins interact in yeast. B, schematic representation of the domain structure of Bif-1 and mapping of the Src binding domain of Bif-1. LexA-c-Src was transfected into yeast with the indicated deletion mutants of Bif-1 fused to B42. Interactions of pairs of fusion proteins were determined by visual examination of β-galactosidase activity and are represented graphically. SH3, Src homology 3. AD, transactivation domain; BD, DNA binding domain; H0, helix zero; H1I, helix one insert, N-BAR, N-terminal bin/amphiphysin/rvs. C, 293T cells were transiently transfected with pFLAG-tagged c-Src or c-Src531 or parental pFLAG-CMV2 vector for 2 days. Immunoprecipitation was performed using anti-FLAG antibody or anti-Myc (control) followed by SDS-PAGE/immunoblot analysis with anti-Bif-1 polyclonal antiserum. D, 3Y1 rat fibroblasts stably expressing c-Src or NIH3T3 cells stably expressing a constitutively active c-Src (Y527F) were subjected to immunoprecipitation with anti-c-Src or control antibody followed by SDS-PAGE/immunoblot analysis.